Infect Chemother.
2008 Dec;40(6):305-310. 10.3947/ic.2008.40.6.305.
Utility of QuantiFERON-TB In-Tube Test for Differentiating Active Tuberculosis from Latent Tuberculosis Infection in an Intermediate Burden Country
- Affiliations
-
- 1Department of Internal Medicine, KEPCO Medical Foundation Hanil General Hospital, Korea.
- 2Department of Internal Medicine and AIDS Research Institute, Yonsei University College of Medicine, Seoul, Korea. D.jmkim@yuhs.ac
- KMID: 1782295
- DOI: http://doi.org/10.3947/ic.2008.40.6.305
Abstract
-
BACKGROUND: The aim of the present study was to assess the contribution of a QuantiFERON-TB Gold In-Tube test (QFT-IT) in differentiating active tuberculosis (TB) from latent tuberculosis infection (LTBI) by quantifying interferon-gamma levels.
MATERIALS AND METHODS
We retrospectively reviewed clinical records of 314 patients older than 15 years who had performed QFT-IT between July 2006 and August 2007 at a tertiary care teaching hospital.
RESULTS
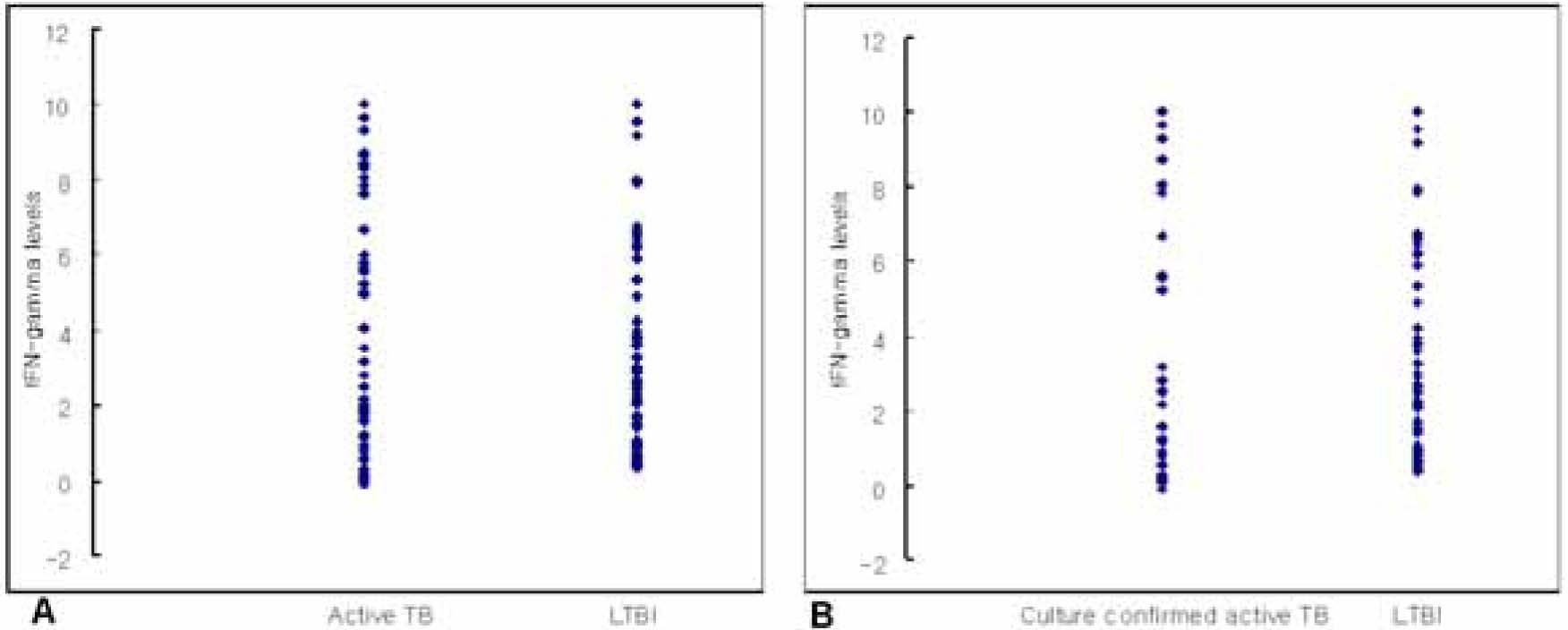
Subjects with active TB (n=81, culture confirmed active TB in 40 subjects) and LTBI (n=76) were included. Mean+/-SD IFN-gamma levels were 4.96+/-3.98 IU/mL (range -0.08-10) for all subjects with active TB, 4.54+/-4.05 IU/mL (range -0.08-10) for culture confirmed active TB, and 4.11+/-3.57 IU/mL (range 0.35-10) for subjects with LTBI. The quantitative results of QFT-IT on IFN-gamma levels between all the subjects with active TB and those with LTBI were not statistically significant (P=0.16). The result was similar when compared between those with culture confirmed active TB and those with LTBI, showing little statistical significance (P=0.554).
CONCLUSION
The production of IFN-gamma measured by QFT-IT showed no correlation between its level and the activity of Mycobacterium tuberculosis infection. These results suggest that measuring IFN-gamma using QFT-IT might not be useful for distinguishing active TB from LTBI.
Keyword
MeSH Terms
Figure
Reference
-
1. Richeldi L. An update on the diagnosis of tuberculosis infection. Am J Respir Crit Care Med. 2006. 174:736–742.
Article2. Diel R, Ernst M, Doscher G, Visuri-Karbe L, Greinert U, Niemann S, Nienhaus A, Lange C. Avoiding the effect of BCG vaccination in detecting Mycobacterium tuberculosis infection with a blood test. Eur Respir J. 2006. 28:16–23.
Article3. Brock I, Weldingh K, Lillebaek T, Follmann F, Andersen P. Comparison of tuberculin skin test and new specific blood test in tuberculosis contacts. Am J Respir Crit Care Med. 2004. 170:65–69.
Article4. Ewer K, Deeks J, Alvarez L, Bryant G, Waller S, Andersen P, Monk P, Lalvani A. Comparison of T-cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet. 2003. 361:1168–1173.
Article5. Lalvani A, Pathan AA, Durkan H, Wilkinson KA, Whelan A, Deeks JJ, Reece WH, Latif M, Pasvol G, Hill AV. Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Lancet. 2001. 357:2017–2021.
Article6. Richeldi L, Ewer K, Losi M, Hansell DM, Roversi P, Fabbri LM, Lalvani A. Early diagnosis of subclinical multidrug-resistant tuberculosis. Ann Intern Med. 2004. 140:709–713.
Article7. Lalvani A, Nagvenkar P, Udwadia Z, Pathan AA, Wilkinson KA, Shastri JS, Ewer K, Hill AV, Mehta A, Rodrigues C. Enumeration of T cells specific for RD1-encoded antigens suggests a high prevalence of latent Mycobacterium tuberculosis infection in healthy urban Indians. J Infect Dis. 2001. 183:469–477.
Article8. Mori T, Sakatani M, Yamagishi F, Takashima T, Kawabe Y, Nagao K, Shigeto E, Harada N, Mitarai S, Okada M, Suzuki K, Inoue Y, Tsuyuguchi K, Sasaki Y, Mazurek GH, Tsuyuguchi I. Specific detection of tuberculosis infection: an interferon-gamma-based assay using new antigens. Am J Respir Crit Care Med. 2004. 170:59–64.9. Kang YA, Lee HW, Yoon HI, Cho B, Han SK, Shim YS, Yim JJ. Discrepancy between the tuberculin skin test and the whole-blood interferon gamma assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA. 2005. 293:2756–2761.
Article10. Mazurek GH, Jereb J, Lobue P, Iademarco MF, Metchock B, Vernon A. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005. 54:49–55.11. Meier T, Eulenbruch HP, Wrighton-Smith P, Enders G, Regnath T. Sensitivity of a new commercial enzyme-linked immunospot assay (T SPOT-TB) for diagnosis of tuberculosis in clinical practice. Eur J Clin Microbiol Infect Dis. 2005. 24:529–536.
Article12. Liebeschuetz S, Bamber S, Ewer K, Deeks J, Pathan AA, Lalvani A. Diagnosis of tuberculosis in South African children with a T-cell-based assay: a prospective cohort study. Lancet. 2004. 364:2196–2203.
Article13. Lee JY, Choi HJ, Park IN, Hong SB, Oh YM, Lim CM, Lee SD, Koh Y, Kim WS, Kim DS, Kim WD, Shim TS. Comparison of two commercial interferon-gamma assays for diagnosing Mycobacterium tuberculosis infection. Eur Respir J. 2006. 28:24–30.
Article14. Ravn P, Munk ME, Andersen AB, Lundgren B, Lund-gren JD, Nielsen LN, Kok-Jensen A, Anderson P, Weldingh K. Prospective evaluation of a whole-blood test using Mycobacterium tuberculosis-specific antigens ESAT-6 and CFP-10 for diagnosis of active tuberculosis. Clin Diagn Lab Immunol. 2005. 12:491–496.
Article15. Goletti D, Vincenti D, Carrara S, Butera O, Bizzoni F, Bernardini G, Amicosante M, Girardi E. Selected RD1 peptides for active tuberculosis diagnosis: comparison of a gamma interferon whole-blood enzyme-linked immunosorbent assay and an enzyme-linked immunospot assay. Clin Diagn Lab Immunol. 2005. 12:1311–1316.
Article16. Goletti D, Carrara S, Vincenti D, Saltini C, Rizzi EB, Schinina V, Ippolito G, Amicosante M, Girardi E. Accuracy of an immune diagnostic assay based on RD1 selected epitopes for active tuberculosis in a clinical setting: a pilot study. Clin Microbiol Infect. 2006. 12:544–550.
Article17. Janssens JP, Roux-Lombard P, Perneger T, Metzger M, Vivien R, Rochat T. Quantitative scoring of an interferon-gamma assay for differentiating active from latent tuberculosis. Eur Respir J. 2007. 30:722–728.
Article18. Cellestis Ltd. Cat. no. 0599 0201. QuantiFERON-TB Gold (In-Tube Method) package insert. 2004. Victoria, Australia:19. WHO. WHO REPORT 2007; Global Tuberculosis Control, Surveillance, Planning, Financing. 2007.20. Kang YA, Lee HW, Hwang SS, Um SW, Han SK, Shim YS, Yim JJ. Usefulness of whole-blood interferon-gamma assay and interferon-gamma enzyme-linked immunospot assay in the diagnosis of active pulmonary tuberculosis. Chest. 2007. 132:959–965.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application of the QuantiFERON(R)-TB Gold Test in Two Cases of Erythema Induratum of Bazin
- The Diagnostic Value of Interferon-gamma Assay in Patients with Active Tuberculosis
- Tuberculosis Infection and Latent Tuberculosis
- Tuberculin Skin Test and QuantiFERON-TB Gold Assay before and after Treatment for Latent Tuberculosis Infection among Health Care Workers in Local Tertiary Hospital
- Comparison of Interferon-gamma Assays with the Tuberculin Skin Test in Children