J Korean Med Sci.
2011 Feb;26(2):214-221. 10.3346/jkms.2011.26.2.214.
Could HBx Protein Expression Affect Signal Pathway Inhibition by Gefitinib or Selumetinib, a MEK Inhibitor, in Hepatocellular Carcinoma Cell Lines?
- Affiliations
-
- 1Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. kimkm70@amc.seoul.kr
- 2Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 1782109
- DOI: http://doi.org/10.3346/jkms.2011.26.2.214
Abstract
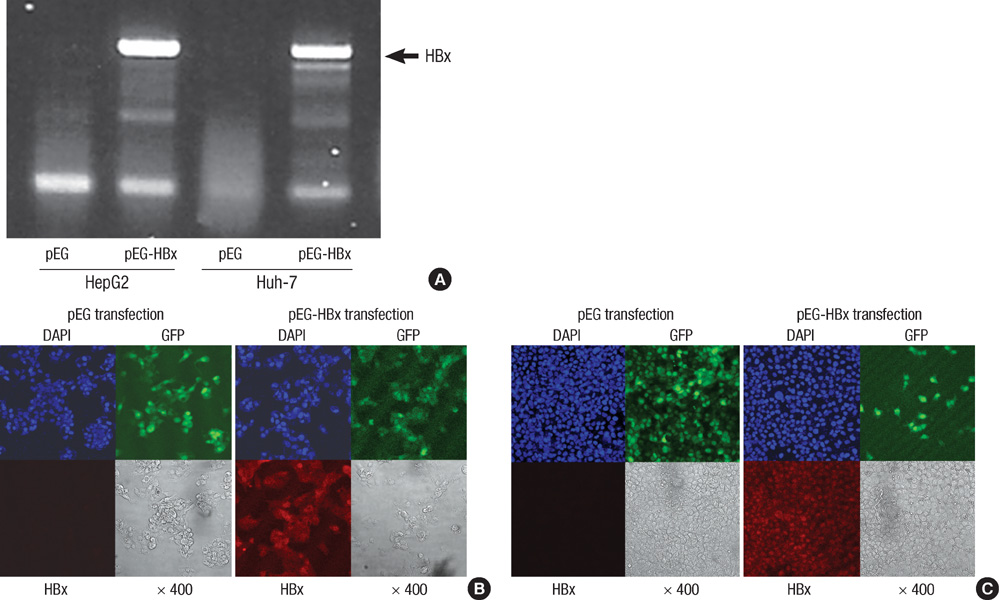
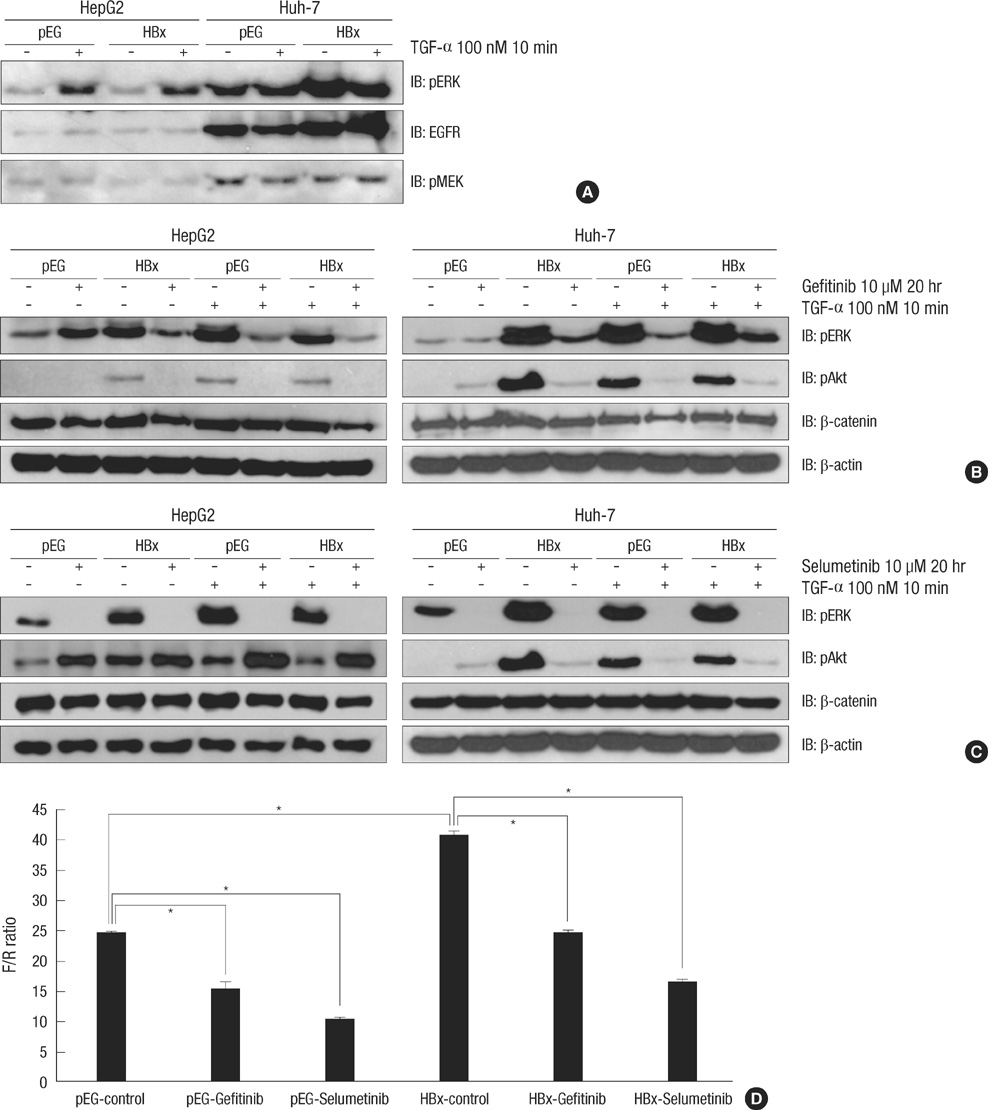
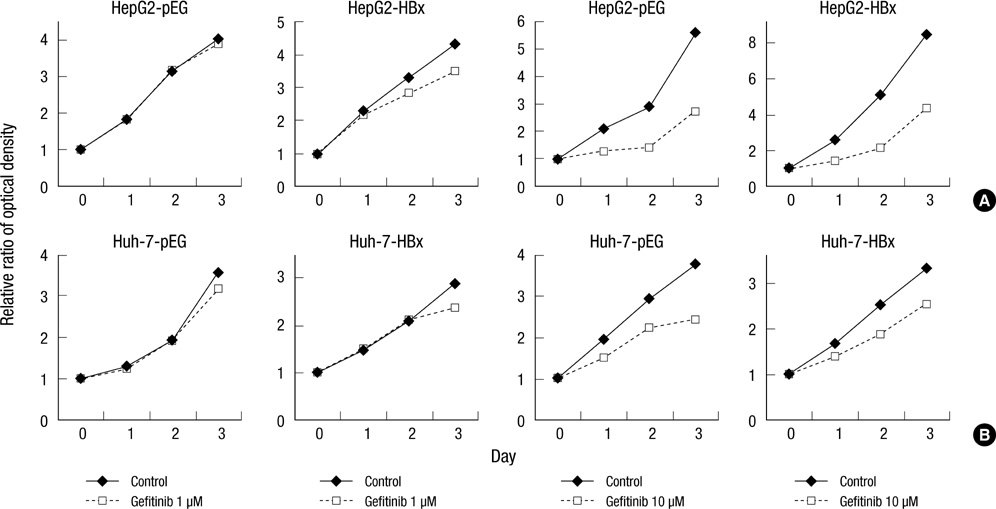
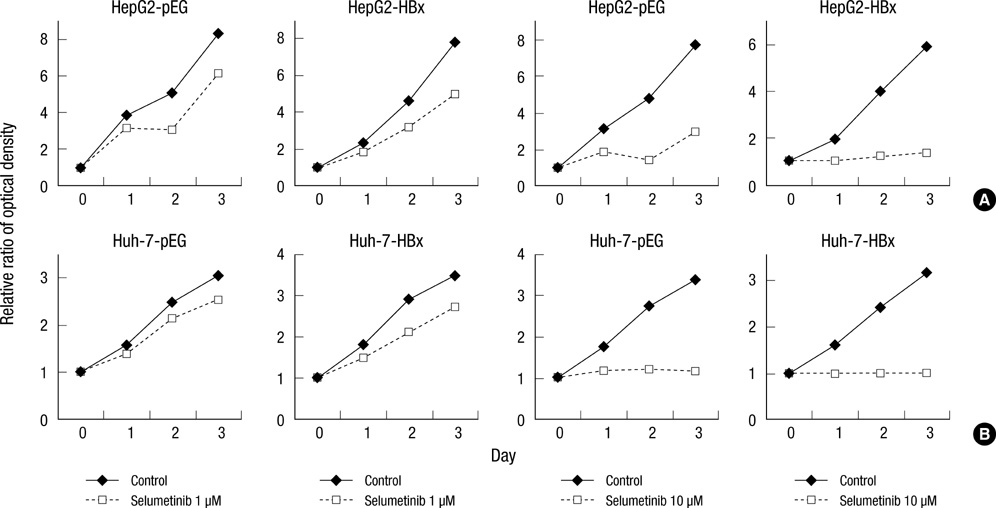
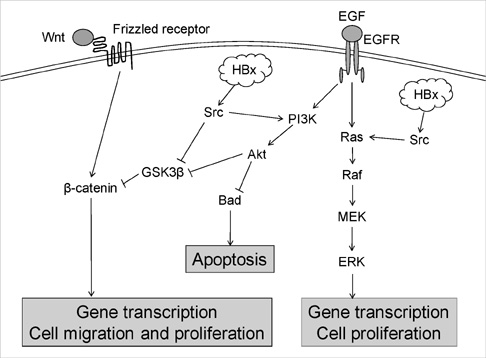
- Hepatitis B virus X (HBx) protein has been known to play an important role in development of hepatocellular carcinoma (HCC). The aim of this study is to find out whether HBx protein expression affects antiproliferative effect of an epidermal growth factor receptor-tyrosine kinase (EGFR-TK) inhibitor and a MEK inhibitor in HepG2 and Huh-7 cell lines. We established HepG2 and Huh-7 cells transfected stably with HBx gene. HBx protein expression increased pERK and pAkt expression as well as beta-catenin activity in both cells. Gefitinib (EGFR-TK inhibitor) inhibited pERK and pAkt expression and beta-catenin activity in both cells. Selumetinib (MEK inhibitor) reduced pERK level and beta-catenin activity but pAkt expression was rather elevated by selumetinib in these cells. Reduction of pERK levels was much stronger with selumetinib than gefitinib in both cells. The antiproliferative efficacy of selumetinib was more potent than that of gefitinib. However, the antiproliferative effect of gefitinib, as well as selumetinib, was not different between cell lines with or without HBx expression. Signal pathway activation by HBx might not be strong enough to attenuate the antiproliferative effect of EGFR-TK inhibitor. Future experiments are needed to understand the role of HBx protein expression in HCC treatment using molecular targeting agent.
Keyword
MeSH Terms
-
Animals
Antineoplastic Agents/*pharmacology
Benzimidazoles/*pharmacology
Carcinoma, Hepatocellular/*metabolism
Cell Line, Tumor/drug effects
Cell Proliferation
Extracellular Signal-Regulated MAP Kinases/metabolism
Humans
Liver Neoplasms/*metabolism
Mitogen-Activated Protein Kinase Kinases/*antagonists & inhibitors
Protein Kinase Inhibitors/*pharmacology
Proto-Oncogene Proteins c-akt
Quinazolines/*pharmacology
Receptor, Epidermal Growth Factor/antagonists & inhibitors
Signal Transduction/*drug effects
Trans-Activators/*metabolism
beta Catenin/metabolism
Figure
Reference
-
1. El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999. 340:745–750.2. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003. 362:1907–1917.3. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003. 37:429–442.4. Bosch FX, Ribes J, Borràs J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999. 19:271–285.5. Liaw YF, Tai DI, Chu CM, Lin DY, Sheen IS, Chen TJ, Pao CC. Early detection of hepatocellular carcinoma in patients with chronic type B hepatitis: a prospective study. Gastroenterology. 1986. 90:263–267.6. Branda M, Wands JR. Signal transduction cascades and hepatitis B and C related hepatocellular carcinoma. Hepatology. 2006. 43:891–902.7. Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, Bartholomeusz G, Li Y, Pan Y, Li Z, Bargou RC, Qin J, Lai CC, Tsai FJ, Tsai CH, Hung MC. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell. 2005. 19:159–170.8. Cha MY, Kim CM, Park YM, Ryu WS. Hepatitis B virus X protein is essential for the activation of Wnt/beta-catenin signaling in hepatoma cells. Hepatology. 2004. 39:1683–1693.9. Benn J, Schneider RJ. Hepatitis B virus HBx protein activates Ras-GTP complex formation and establishes a Ras, Raf, MAP kinase signaling cascade. Proc Natl Acad Sci U S A. 1994. 91:10350–10354.10. Shih WL, Kuo ML, Chuang SE, Cheng AL, Doong SL. Hepatitis B virus X protein activates a survival signaling by linking SRC to phosphatidylinositol 3-kinase. J Biol Chem. 2003. 278:31807–31813.11. Chung TW, Lee YC, Kim CH. Hepatitis B viral HBx induces matrix metalloproteinase-9 gene expression through activation of ERK and PI-3K/AKT pathways: involvement of invasive potential. FASEB J. 2004. 18:1123–1125.12. Villanueva A, Newell P, Chiang DY, Friedman SL, Llovet JM. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis. 2007. 27:55–76.13. Höpfner M, Sutter AP, Huether A, Schuppan D, Zeitz M, Scherübl H. Targeting the epidermal growth factor receptor by gefitinib for treatment of hepatocellular carcinoma. J Hepatol. 2004. 41:1008–1016.14. Huether A, Höpfner M, Sutter AP, Schuppan D, Scherübl H. Erlotinib induces cell cycle arrest and apoptosis in hepatocellular cancer cells and enhances chemosensitivity towards cytostatics. J Hepatol. 2005. 43:661–669.15. Schiffer E, Housset C, Cacheux W, Wendum D, Desbois-Mouthon C, Rey C, Clergue F, Poupon R, Barbu V, Rosmorduc O. Gefitinib, an EGFR inhibitor, prevents hepatocellular carcinoma development in the rat liver with cirrhosis. Hepatology. 2005. 41:307–314.16. Okano J, Matsumoto K, Nagahara T, Murawaki Y. Gefitinib and the modulation of the signaling pathways downstream of epidermal growth factor receptor in human liver cancer cells. J Gastroenterol. 2006. 41:166–176.17. Klein NP, Schneider RJ. Activation of Src family kinases by hepatitis B virus HBx protein and coupled signaling to Ras. Mol Cell Biol. 1997. 17:6427–6436.18. Miao J, Chen GG, Chun SY, Lai PP. Hepatitis B virus X protein induces apoptosis in hepatoma cells through inhibiting Bcl-xL expression. Cancer Lett. 2006. 236:115–124.19. Alpini G, Phillips JO, Vroman B, LaRusso NF. Recent advances in the isolation of liver cells. Hepatology. 1994. 20:494–514.20. Pang RW, Poon RT. From molecular biology to targeted therapies for hepatocellular carcinoma: the future is now. Oncology. 2007. 72:Suppl 1. 30–44.21. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J. SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008. 359:378–390.22. Huynh H, Soo KC, Chow PK, Tran E. Targeted inhibition of the extracellular signal-regulated kinase kinase pathway with AZD6244 (ARRY-142886) in the treatment of hepatocellular carcinoma. Mol Cancer Ther. 2007. 6:138–146.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pro-apoptotic function of hepatitis B virus X protein
- Growth inhibition in head and neck cancer cell lines by gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor
- C-terminal-truncated HBV X promotes hepato-oncogenesis through inhibition of tumor-suppressive β-catenin/BAMBI signaling
- Hepatitis B virus X Protein Promotes Liver Cancer Progression through Autophagy Induction in Response to TLR4 Stimulation
- Hepatitis B virus X Protein Promotes Liver Cancer Progression through Autophagy Induction in Response to TLR4 Stimulation