J Korean Med Sci.
2009 Oct;24(5):918-929. 10.3346/jkms.2009.24.5.918.
DNA Methylation and Expression Patterns of Key Tissue-specific Genes in Adult Stem Cells and Stomach Tissues
- Affiliations
-
- 1Department of Microbiology, College of Medicine, The Catholic University of Korea, Seoul, Korea. rhyumung@catholic.ac.kr
- 2Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Department of Surgery, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 4Department of Clinical Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 1782016
- DOI: http://doi.org/10.3346/jkms.2009.24.5.918
Abstract
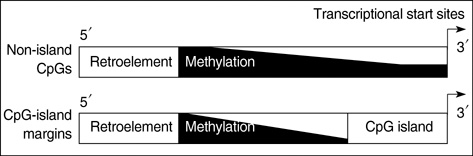
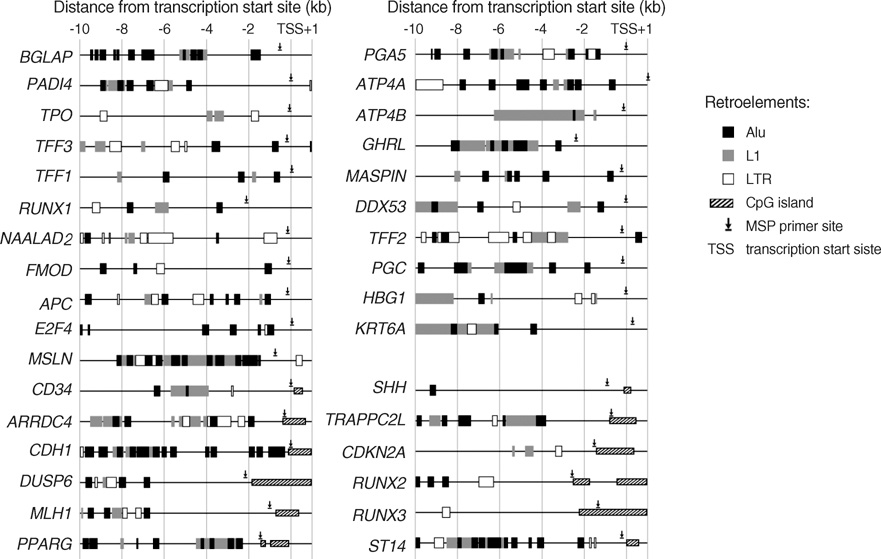
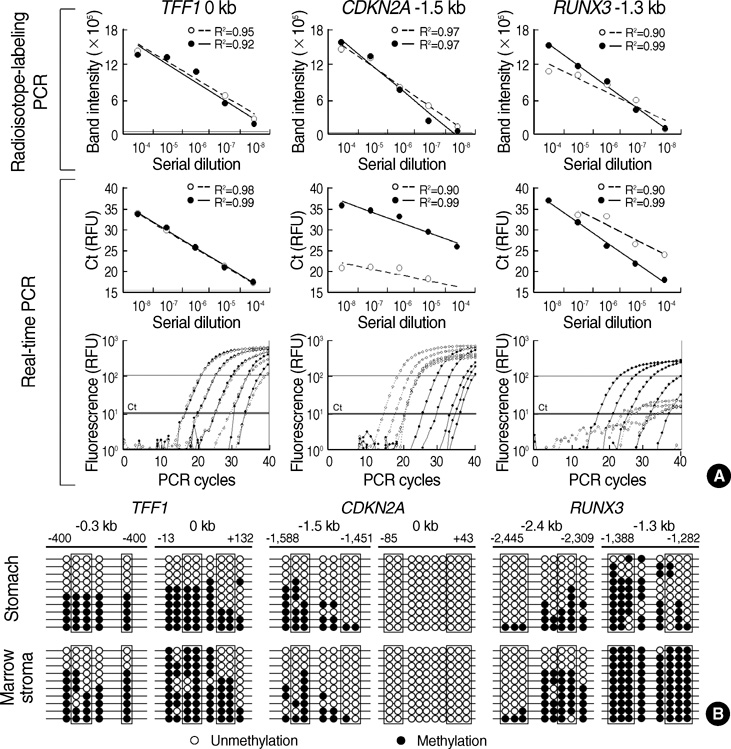
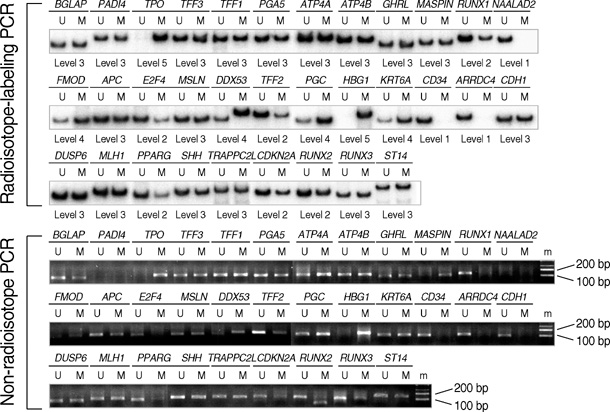
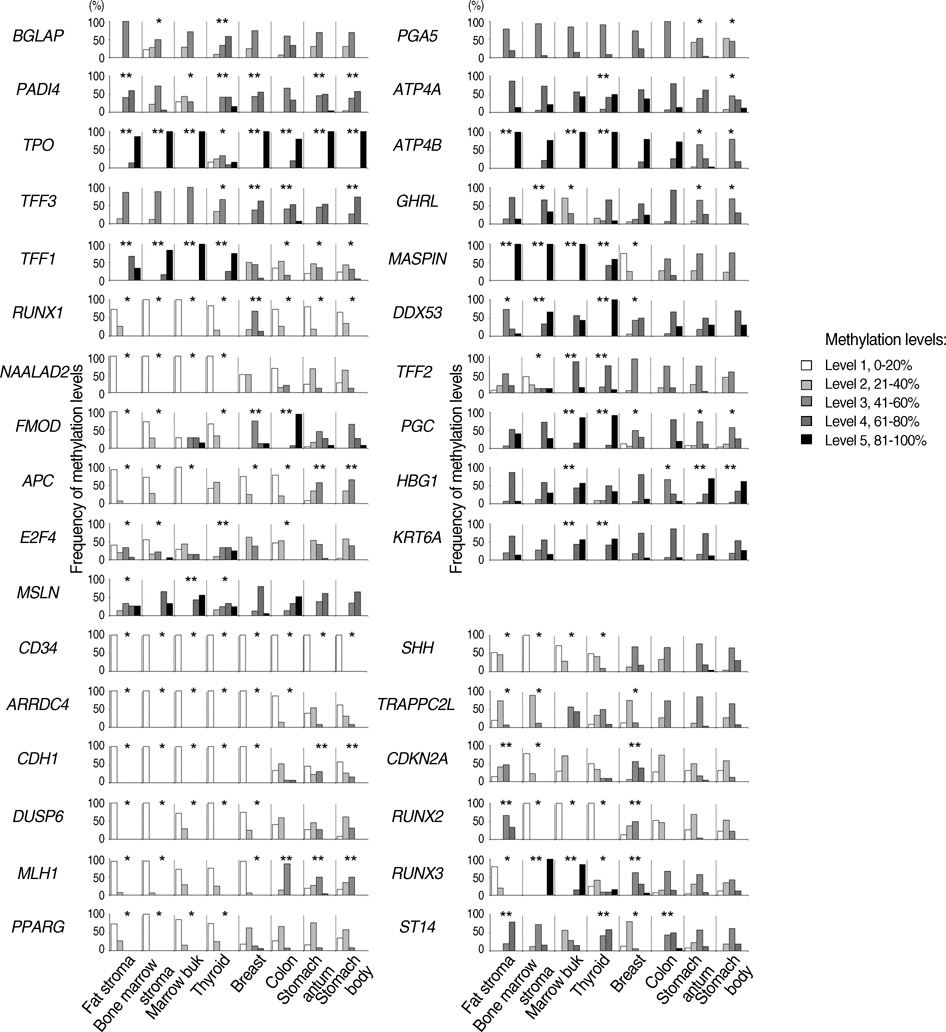
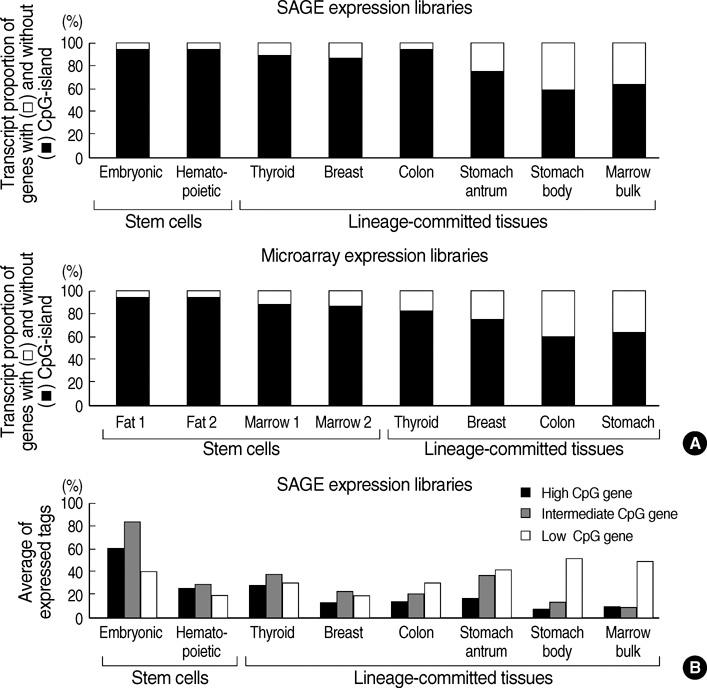
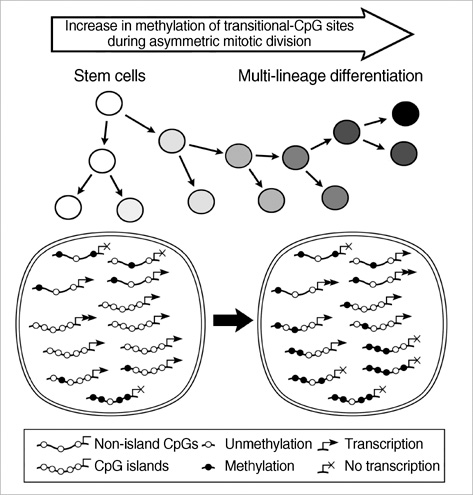
- CpG-island margins and non-island-CpG sites round the transcription start sites of CpG-island-positive and -negative genes are methylated to various degrees in a tissue-specific manner. These methylation-variable CpG sites were analyzed to delineate a relationship between the methylation and transcription of the tissue-specific genes. The level of tissue-specific transcription was estimated by counting the number of the total transcripts in the SAGE (serial analysis of gene expression) database. The methylation status of 12 CpG-island margins and 21 non-island CpG sites near the key tissue-specific genes was examined in pluripotent stromal cells obtained from fat and bone marrow samples as well as in lineage-committed cells from marrow bulk, stomach, colon, breast, and thyroid samples. Of the 33 CpG sites examined, 10 non-island-CpG sites, but none of the CpG-island margins were undermethylated concurrent with tissue-specific expression of their nearby genes. The net methylation of the 33 CpG sites and the net amount of non-island-CpG gene transcripts were high in stomach tissues and low in stromal cells. The present findings suggest that the methylation of the non-island-CpG sites is inversely associated with the expression of the nearby genes, and the concert effect of transitional-CpG methylation is linearly associated with the stomach-specific genes lacking CpG-islands.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
DNA Methylation Patterns of Ulcer-Healing Genes Associated with the Normal Gastric Mucosa of Gastric Cancers
Seung-Jin Hong, Jung-Hwan Oh, Yu-Chae Jung, Young-Ho Kim, Sung-Ja Kim, Seok-Jin Kang, Eun-Joo Seo, Sang-Wook Choi, Moo-Il Kang, Mun-Gan Rhyu
J Korean Med Sci. 2010;25(3):405-417. doi: 10.3346/jkms.2010.25.3.405.Gastric Mucosal Atrophy Impedes Housekeeping Gene Methylation in Gastric Cancer Patients
Jung-Hwan Oh, Mun-Gan Rhyu, Suk-Il Kim, Mi-Ri Yun, Jung-Ha Shin, Seung-Jin Hong
Cancer Res Treat. 2019;51(1):267-279. doi: 10.4143/crt.2018.085.
Reference
-
1. Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet. 2002. 3:662–673.
Article2. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001. 7:211–228.
Article3. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002. 418:41–49.
Article4. Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004. 306:1568–1571.
Article5. Yatabe Y, Tavare S, Shibata D. Investigating stem cells in human colon by using methylation patterns. Proc Natl Acad Sci U S A. 2001. 98:10839–10844.
Article6. Eckhardt F, Lewin J, Cortese R, Rakyan VK, Attwood J, Burger M, Burton J, Cox TV, Davies R, Down TA, Haefliger C, Horton R, Howe K, Jackson DK, Kunde J, Koenig C, Liddle J, Niblett D, Otto T, Pettett R, Seemann S, Thompson C, West T, Rogers J, Olek A, Berlin K, Beck S. DNA methylation profiling of human chromosomes 6, 20 and 22. Nat Genet. 2006. 38:1378–1385.
Article7. Weber M, Hellmann I, Stadler MB, Ramos L, Paabo S, Rebhan M, Schubeler D. Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat Genet. 2007. 39:457–466.
Article8. Larsen F, Gundersen G, Lopez R, Prydz H. CpG islands as gene markers in the human genome. Genomics. 1992. 13:1095–1107.
Article9. Noer A, Sorensen AL, Boquest AC, Collas P. Stable CpG hypomethylation of adipogenic promoters in freshly isolated, cultured, and differentiated mesenchymal stem cells from adipose tissue. Mol Biol Cell. 2006. 17:3543–3556.
Article10. Kang MI, Kim HS, Jung YC, Kim YH, Hong SJ, Kim MK, Baek KH, Kim CC, Rhyu MG. Transitional CpG methylation between promoters and retroelements of tissue-specific genes during human mesenchymal cell differentiation. J Cell Biochem. 2007. 102:224–239.
Article11. Medstrand P, van de Lagemaat LN, Mager DL. Retroelement distributions in the human genome: variations associated with age and proximity to genes. Genome Res. 2002. 12:1483–1495.
Article12. Arnaud P, Goubely C, Pelissier T, Deragon JM. SINE retroposons can be used in vivo as nucleation centers for de novo methylation. Mol Cell Biol. 2000. 20:3434–3441.
Article13. Turker MS. Gene silencing in mammalian cells and the spread of DNA methylation. Oncogene. 2002. 21:5388–5393.
Article14. Kang MI, Rhyu MG, Kim YH, Jung YC, Hong SJ, Cho CS, Kim HS. The length of CpG islands is associated with the distribution of Alu and L1 retroelements. Genomics. 2006. 87:580–590.
Article15. Kim YH, Hong SJ, Jung YC, Kim SJ, Seo EJ, Choi SW, Rhyu MG. The 5'-end transitional CpGs between the CpG islands and retroelements are hypomethylated in association with loss of heterozygosity in gastric cancers. BMC Cancer. 2006. 6:180.
Article16. Jung YC, Hong SJ, Kim YH, Kim SJ, Kang SJ, Choi SW, Rhyu MG. Chromosomal losses are associated with hypomethylation of the gene-control regions in the stomach with a low number of active genes. J Korean Med Sci. 2008. 23:1068–1089.
Article17. Hong SJ, Kim YH, Choi YD, Min KO, Choi SW, Rhyu MG. Relationship between the extent of chromosomal losses and the pattern of CpG methylation in gastric carcinomas. J Korean Med Sci. 2005. 20:790–805.
Article18. Misteli T. Beyond the sequence: cellular organization of genome function. Cell. 2007. 128:787–800.
Article19. Chakalova L, Debrand E, Mitchell JA, Osborne CS, Fraser P. Replication and transcription: shaping the landscape of the genome. Nat Rev Genet. 2005. 6:669–677.
Article20. Tagoh H, Melnik S, Lefevre P, Chong S, Riggs AD, Bonifer C. Dynamic reorganization of chromatin structure and selective DNA demethylation prior to stable enhancer complex formation during differentiation of primary hematopoietic cells in vitro. Blood. 2004. 103:2950–2955.
Article21. Rajasekhar VK, Begemann M. Concise review: roles of polycomb group proteins in development and disease: a stem cell perspective. Stem Cells. 2007. 25:2498–2510.
Article22. Yoo EJ, Park SY, Cho NY, Kim N, Lee HS, Kang GH. Helicobacter pylori-infection-associated CpG island hypermethylation in the stomach and its possible association with Polycomb repressive marks. Virchows Arch. 2008. 452:515–524.
Article23. Van De Bovenkamp JH, Korteland-Van Male AM, Buller HA, Einerhand AW, Dekker J. Infection with Helicobacter pylori affects all major secretory cell populations in the human antrum. Dig Dis Sci. 2005. 50:1078–1086.
Article24. Morgan HD, Santos F, Green K, Dean W, Reik W. Epigenetic reprogramming in mammals. Hum Mol Genet. 2005. 14(Spec No 1):R47–R58.
Article25. Beckmann J, Scheitza S, Wernet P, Fischer JC, Giebel B. Asymmetric cell division within the human hematopoietic stem and progenitor cell compartment: identification of asymmetrically segregating proteins. Blood. 2007. 109:5494–5501.
Article26. Kiel MJ, He S, Ashkenazi R, Gentry SN, Teta M, Kushner JA, Jackson TL, Morrison SJ. Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature. 2007. 449:238–242.
Article27. Easwaran HP, Schermelleh L, Leonhardt H, Cardoso MC. Replication-independent chromatin loading of Dnmt1 during G2 and M phases. EMBO Rep. 2004. 5:1181–1186.
Article28. Lin H, Gupta V, Vermilyea MD, Falciani F, Lee JT, O'Neill LP, Turner BM. Dosage compensation in the mouse balances up-regulation and silencing of X-linked genes. PLoS Biol. 2007. 5:326.
Article29. Tusnady GE, Simon I, Varadi A, Aranyi T. BiSearch: primer-design and search tool for PCR on bisulfite-treated genomes. Nucleic Acids Res. 2005. 33:9.