J Korean Med Sci.
2006 Apr;21(2):272-278. 10.3346/jkms.2006.21.2.272.
Expression of Angiopoietin 1, 2 and Their Common Receptor Tie2 in Human Gastric Carcinoma: Implication for Angiogenesis
- Affiliations
-
- 1Department of Pathology, Chonbuk National University, Medical School, Institute for Medical Sciences and Center for Healthcare Technology Development, Jeonju, Korea. mws@chonbuk.ac.kr
- 2Department of Medicine, Veterans Administration Medical Center, Long Beach, California, and the University of California, Irvine, California, U.S.A.
- KMID: 1781834
- DOI: http://doi.org/10.3346/jkms.2006.21.2.272
Abstract
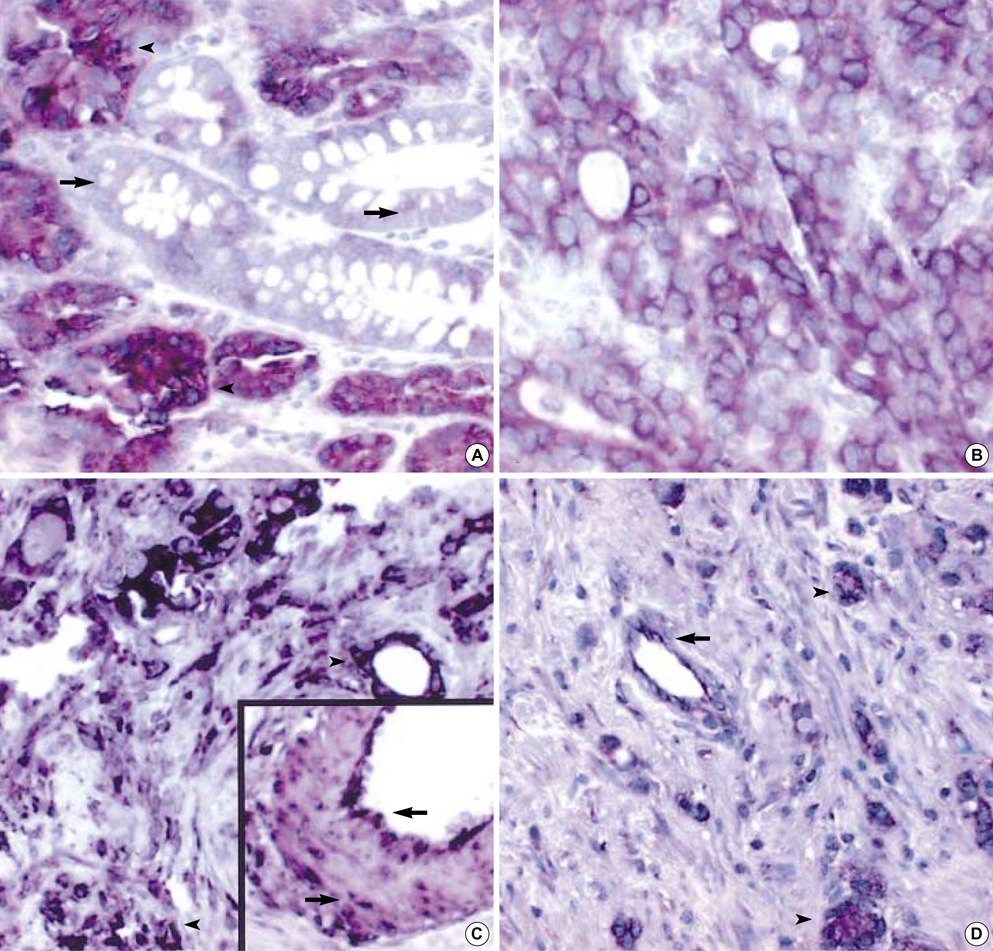
- Angiogenesis, formation of new microvessels providing oxygen and nutrient supply, is essential for tumor growth. It is dependent on the production of angiogenic growth factors by tumor cells. Angiopoietin 1 (Ang-1) and 2 (Ang-2) and their common receptor, Tie2, are thought to be critical regulators of tumor angiogenesis. We examined expression of Ang-1, Ang-2, and their common receptor Tie2 mRNAs and proteins in gastric cancers using in situ hybridization and immunohistochemistry. We also investigated the relationship between their expression and differentiation of cancer cells, lymph node metastasis, tumor size, depth of cancer cell invasion, TNM staging and microvessel density (MVD). The expression of Ang-1, Ang-2, and Tie2 mRNA in cancer cells significantly correlated with the MVD (p<0.001, <0.001 and =0.019, respectively). Ang-1 and Tie2 positivity correlated with advanced gastric cancers (p<0.05) and larger cancers had higher positive rates of Ang-1, Ang-2, and Tie2 mRNA expression (p<0.001, =0.010 and =0.039, respectively). Significant positive correlations were also found between mRNA expression of Tie2 and those of Ang-1 and Ang-2 (p<0.01 and <0.001, respectively). These findings indicate that the expression of Ang-1 and Ang-2 is important for tumor angiogenesis, and suggest a possible role of autocrine/paracrine function of angiopoietin/Tie2 system in gastric cancer progression.
Keyword
MeSH Terms
-
Stomach Neoplasms/blood supply/*genetics/*metabolism/pathology
Receptor, TIE-2/*genetics/*metabolism
RNA, Neoplasm/genetics/metabolism
RNA, Messenger/genetics/metabolism
Neovascularization, Pathologic
Middle Aged
Male
In Situ Hybridization
Immunohistochemistry
Humans
Gene Expression
Female
Carcinoma, Signet Ring Cell/blood supply/genetics/metabolism/pathology
Angiopoietin-2/*genetics/*metabolism
Angiopoietin-1/*genetics/*metabolism
Aged
Adult
Adenocarcinoma/blood supply/genetics/metabolism/pathology
Figure
Reference
-
1. Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990. 82:4–6.
Article2. Fox SB, Gatter KC, Harris AL. Tumor angiogenesis. J Pathol. 1996. 179:232–237.3. Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996. 87:1161–1169.
Article4. Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996. 87:1171–1180.
Article5. Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997. 277:55–60.
Article6. Dumont DJ, Gradwohl GJ, Fong GH, Auerbach R, Breitman ML. The endothelial-specific receptor tyrosine kinase, tek, is a member of a new subfamily of receptors. Oncogene. 1993. 8:1293–1301.7. Runting AS, Stacker SA, Wilks AF. Tie2, a putative protein tyrosine kinase from a new class of cell surface receptor. Growth Factors. 1993. 9:99–105.8. Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, Sato TN, Yancopoulos GD. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998. 282:468–471.
Article9. Papapetropoulos A, Garcia-Cardena G, Dengler TJ, Maisonpierre PC, Yancopoulos GD, Sessa WC. Direct actions of angiopoietin-1 on human endothelium: evidence for network stabilization, cell survival, and interaction with other angiogenic growth factors. Lab Invest. 1999. 79:213–223.10. Bachelder RE, Crago A, Chung J, Wendt MA, Shaw LM, Robinson G, Mercurio AM. Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast cancer cells. Cancer Res. 2001. 61:5736–5740.11. Saito H, Tsujitani S, Oka S, Kondo A, Ikeguchi M, Maeta M, Kaibara N. The expression of transforming growth factor-β1 is significantly correlated with the expression of vascular endothelial growth factor and poor prognosis of patients with advanced gastric carcinoma. Cancer. 1999. 86:1455–1462.
Article12. Maeda K, Kang SM, Ogawa M, Onoda N, Sawada T, Nakata B, Kato Y, Chung YS, Sowa M. Combined analysis of vascular endothelial growth factor and platelet-derived endothelial cell growth factor expression in gastric carcinoma. Int J Cancer. 1997. 74:545–550.
Article13. Maeda K, Kang SM, Onoda N, Ogawa M, Sawada T, Nakata B, Kato Y, Chung YS, Sowa M. Expression of p53 and vascular endothelial growth factor associated with tumor angiogenesis and prognosis in gastric cancer. Oncology. 1998. 55:594–599.
Article14. Shaw JP, Basch R, Shamamian P. Hematopoietic stem cells and endothelial cell precursors express Tie-2, CD31 and CD45. Blood Cells Mol Dis. 2004. 32:168–175.
Article15. Muller A, Lange K, Gaiser T, Hofmann M, Bartels H, Feller AC, Merz H. Expression of angiopoietin-1 and its receptor TEK in hematopoietic cells from patients with myeloid leukemia. Leuk Res. 2002. 26:163–168.16. Wurmbach JH, Hammerer P, Sevinc S, Huland H, Ergun S. The expression of angiopoietins and their receptor Tie-2 in human prostate carcinoma. Anticancer Res. 2000. 20:5217–5220.17. Japanese Research Society for Gastric Cancer. Japanese classification of gastric carcinoma. 1995. Tokyo: Kanehara & Co. Ltd..18. American Joint Committee on Cancer. Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M, editors. Stomach. AJCC cancer staging manual. 2002. 6th ed. New York, NY: Springer Publishers;99–103.19. Fenoglio CM, Noffsinger AE, Stemmermass GN, Lantz PE, Listrom MB, Rilke FO, editors. The Neoplastic Stomach. Gastrointestinal Pathology An Atlas and Text. 1999. 2nd ed. Philadelphia, NY: Lippincott-Raven Publishers;265.20. Hayes AJ, Huang WQ, Yu J, Maisonpierre PC, Liu A, Kern FG, Lippman ME, McLeskey SW, Li LY. Expression and function of angiopoietin-1 in breast cancer. Br J Cancer. 2000. 83:1154–1160.
Article21. Ahmad SA, Liu W, Jung YD, Fan F, Wilson M, Reinmuth N, Shaheen RM, Bucana CD, Ellis LM. The effects of angiopoietin-1 and -2 on tumor growth and angiogenesis in human colon cancer. Cancer Res. 2001. 61:1255–1259.22. Machein MR, Knedla A, Knoth R, Wagner S, Neuschl E, Plate KH. Angiopoietin-1 promotes tumor angiogenesis in a rat glioma model. Am J Pathol. 2004. 165:1557–1570.
Article23. Torimura T, Ueno T, Kin M, Harada R, Taniguchi E, Nakamura T, Sakata R, Hashimoto O, Sakamoto M, Kumashiro R, Sata M, Nakashima O, Yano H, Kojiro M. Overexpression of angiopoietin-1 and angiopoietin-2 in hepatocellular carcinoma. J Hepatol. 2004. 40:799–807.
Article24. Koga K, Todaka T, Morioka M, Hamada J, Kai Y, Yano S, Okamura A, Takakura N, Suda T, Ushio Y. Expression of angiopoietin-2 in human glioma cells and its role for angiogenesis. Cancer Res. 2001. 61:6248–6254.25. Moon WS, Rhyu KH, Kang MJ, Lee DG, Yu HC, Yeum JH, Koh GY, Tarnawski AS. Overexpression of VEGF and angiopoietin 2: a key to high vascularity of hepatocellular carcinoma? Mod Pathol. 2003. 16:552–557.
Article26. Currie MJ, Gunningham SP, Han C, Scott PA, Robinson BA, Harris AL, Fox SB. Angiopoietin-1 is inversely related to thymidine phosphorylase expression in human breast cancer, indicating a role in vascular remodeling. Clin Cancer Res. 2001. 7:918–927.27. Tanaka F, Ishikawa S, Yanagihara K, Miyahara R, Kawano Y, Li M, Otake Y, Wada H. Expression of angiopoietins and its clinical significance in non-small cell lung cancer. Cancer Res. 2002. 62:7124–7129.28. Etoh T, Inoue H, Tanaka S, Barnard GF, Kitano S, Mori M. Angiopoietin-2 is related to tumor angiogenesis in gastric carcinoma: Possible in vivo regulation via induction of proteases. Cancer Res. 2001. 61:2145–2153.29. Shim WS, Teh M, Mack PO, Ge R. Inhibition of angiopoietin-1 expression in tumor cells by an antisense RNA approach inhibited xenograft tumor growth in immunodeficient mice. Int J Cancer. 2001. 94:6–15.
Article30. Nakashima M, Uchida T, Tsukazaki T, Hamanaka Y, Fukuda E, Ito M, Sekine I. Expression of tyrosine kinase receptors Tie-1 and Tie-2 in giant cell tumor of the tendon sheath: a possible role in synovial proliferation. Pathol Res Pract. 2001. 197:101–107.
Article31. Lehtola L, Partanen J, Sistonen L, Korhonen J, Warri A, Harkonen P, Clarke R, Alitalo K. Analysis of tyrosine kinase mRNAs including four FGF receptor mRNAs expressed in MCF-7 breast-cancer cells. Int J Cancer. 1992. 50:598–603.
Article32. Soker S, Kaefer M, Johnson M, Klagsbrun M, Atala A, Freeman MR. Vascular endothelial growth factor-mediated autocrine stimulation of prostate tumor cells coincides with progression to a malignant phenotype. Am J Pathol. 2001. 159:651–659.
Article33. Dias S, Hattori K, Zhu Z, Heissig B, Choy M, Lane W, Wu Y, Chadburn A, Hyjek E, Gill M, Hicklin DJ, Witte L, Moore MA, Rafii S. Autocrine stimulation of VEGFR-2 activates human leukemic cell growth and migration. J Clin Invest. 2000. 106:511–521.
Article34. Nakayama T, Yoshizaki A, Kawahara N, Ohtsuru A, Wen CY, Fukuda E, Nakashima M, Sekine I. Expression of Tie-1 and 2 receptors, and angiopoietin-1, 2 and 4 in gastric carcinoma; immunohistochemical analyses and correlation with clinicopathologic factors. Histopathology. 2004. 44:232–239.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Study on the Expression of Angiopoietin-1, Angiopoietin-2, and Tie2 in Mouse Kidney Maturation
- Tie2 is tied at the cell-cell contacts and to extracellular matrix by Angiopoietin-1
- VEGF-Angiopoietin-Tie2 System in Diabetic Retinopathy
- Investigating the expression pattern of the angiopoietin-Tie system in ALL and its correlation with baseline characteristics
- Expression and Clinical Significance of Angiopoietin-2 and its Receptor Tie-2 in Invasive Breast Cancer