J Korean Med Sci.
2005 Oct;20(5):829-834. 10.3346/jkms.2005.20.5.829.
Expressions of HSP70 and HSP27 in Hepatocellular Carcinoma
- Affiliations
-
- 1Department of Pathology, Inje University Ilsanpaik Hospital, Goyang, Korea. meejoo@ilsanpaik.ac.kr
- 2Department of Surgery, Inje University Seoul Paik Hospital, Seoul, Korea.
- KMID: 1781767
- DOI: http://doi.org/10.3346/jkms.2005.20.5.829
Abstract
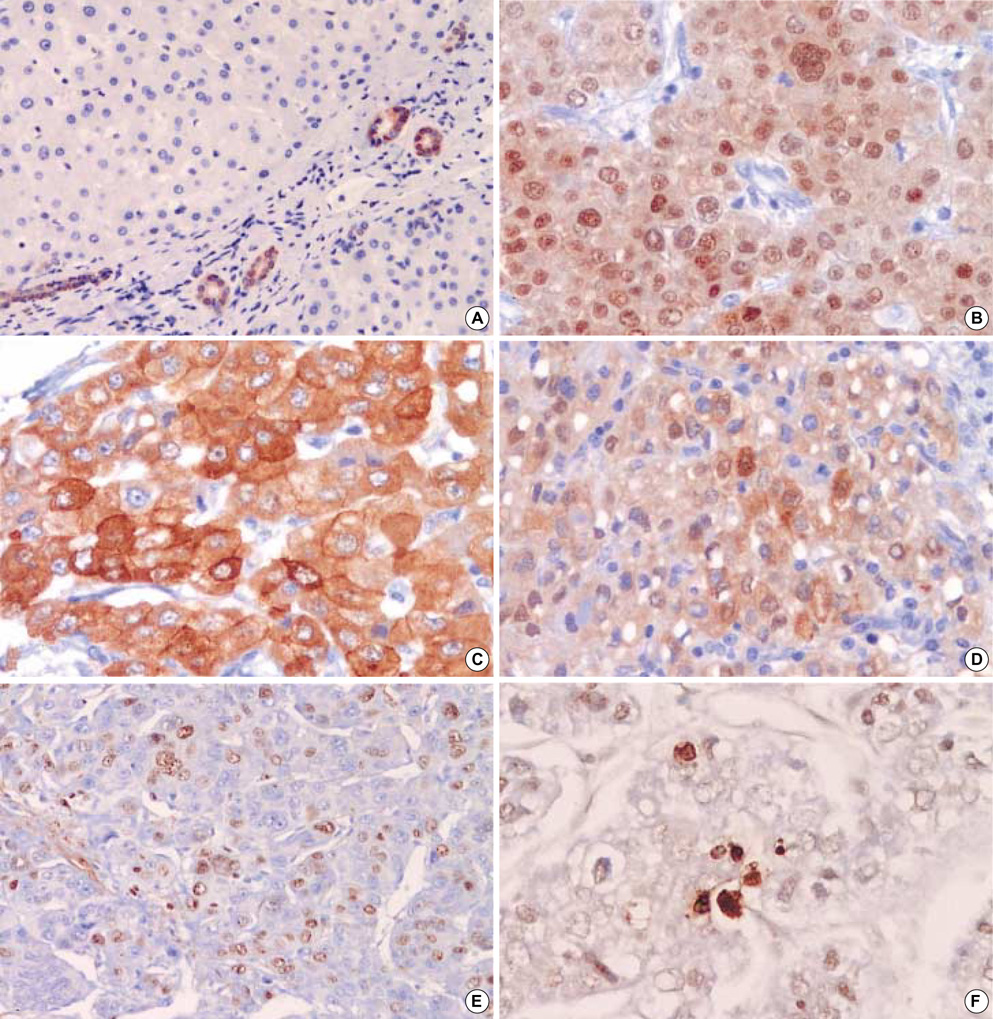
- The heat shock proteins (HSPs) are ubiquitous molecules induced in cells exposed to various stress conditions, including carcinogenesis. The HSP70 and HSP27 among HSPs are of special relevance in human cancer inhibiting apoptosis. The aim of this study is to investigate the expressions of HSP70 and HSP27 in hepatocellular carcinoma (HCC) in association to tumor cell proliferation and apoptosis. We examined the expressions of HSP70 and HSP27 by immunohistochemical staining in 71 cases of HCC, and then related their expressions to clinicopathologic parameters and expressions of p53, Ki-67 and Apotag. HSP70 and HSP27 were frequently stained in the cytoplasm and nuclei of tumor cells, but not in the non-neoplastic hepatocytes. Immunoreactivities of HSP70 and HSP27 were observed in 56.3% and 61.9% of HCCs, respectively. HSP70 immunoreactivity correlated with high Ki-67 labeling indices (LIs) (p=0.0159), large tumor size (p=0.0129), presence of portal vein invasion (p=0.0231), and high tumor stage (p=0.0392). HSP27 immunoreactivity significantly related with the subgroup of HBV-associated HCCs (p=0.0003), but not with the others. Both HSP70 and HSP27 immunoreactivities showed no relation to Apotag LIs or p53 immunoreactivity. In conclusion, expressions of HSP70 and HSP27 may play an important role in hepatocarcinogenesis, and especially HSP70 showed a close relationship to the pathological parameters associated with tumor progression and high Ki-67 LIs. Our results could be additional evidence that HSP70 expressions can contribute to not only hepatocarcinogenesis but also tumor progression by promoting tumor cell proliferation.
Keyword
MeSH Terms
-
Carcinoma, Hepatocellular/*metabolism
Female
Gene Expression Regulation, Neoplastic
HSP70 Heat-Shock Proteins/*metabolism
Heat-Shock Proteins/*metabolism
Humans
Liver Neoplasms/*metabolism
Male
Middle Aged
Neoplasm Proteins/*metabolism
Research Support, Non-U.S. Gov't
Tumor Cells, Cultured
Tumor Markers, Biological/*metabolism
Figure
Cited by 1 articles
-
Expression of the RERG Gene is Gender-Dependent in Hepatocellular Carcinoma and Regulated by Histone Deacetyltransferases
Ai-Guo Wang, Wan Fang, Ying-Hao Han, Sang-Mi Cho, Jong Young Choi, Kee Ho Lee, Wook Hwan Kim, Jin Man Kim, Moon Gi Park, Dae-Yeul Yu, Nam-Soon Kim, Dong-Seok Lee
J Korean Med Sci. 2006;21(5):891-896. doi: 10.3346/jkms.2006.21.5.891.
Reference
-
1. Lindquist S. The heat-shock response. Annu Rev Biochem. 1986. 55:1151–1191.
Article2. Morimoto RI. Cells in stress: transcriptional activation of heat shock genes. Science. 1993. 259:1409–1410.
Article3. Mosser DD, Morimoto RI. Molecular chaperones and the stress of oncogenesis. Oncogene. 2004. 23:2907–2918.
Article4. Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000. 92:1564–1572.
Article5. Helmbrecht K, Zeise E, Rensing L. Chaperones in cell cycle regulation and mitogenic signal transduction: a review. Cell Prolif. 2000. 33:341–365.
Article6. Garrido C, Gurbuxani S, Ravagnan L, Kroemer G. Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem Biophys Res Commun. 2001. 286:433–442.
Article7. Ciocca DR, Clark GM, Tandon AK, Fuqua SA, Welch WJ, McGuire WL. Heat shock protein hsp70 in patients with axillary lymph node-negative breast cancer: prognostic implications. J Natl Cancer Inst. 1993. 85:570–574.
Article8. Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991. 351:453–456.
Article9. Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988. 22:631–677.
Article10. Craig EA, Weissman JS, Horwich AL. Heat shock proteins and molecular chaperones: mediators of protein conformation and turnover in the cell. Cell. 1994. 78:365–372.
Article11. Morimoto RI, Tisseres A, Georgopoulos C. Heat shock proteins in biology and medicine. 1994. Cold Spring Harbor, New York: Cold Spring Harbor Press.12. Scharf KD, Höhfeld I, Nover L. 1998. Heat stress response and heat stress transcription factors. J Biosci. 1998. 23:313–329.13. Nylandsted J, Brand K, Jaattela M. Heat shock protein 70 is required for the survival of cancer cells. Ann N Y Acad Sci. 2000. 926:122–125.
Article14. Mosser DD, Caron AW, Bourget L, Denis-Larose C, Massie B. Role of human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol Cell Biol. 1997. 17:5317–5327.15. Zylicz M, King FW, Wawrzynow A. Hsp70 interactions with the p53 tumour suppressor protein. EMBO J. 2001. 20:4634–4638.16. Malusecka E, Zborek A, Krzyzowska-Gruca S, Krawczyk Z. Expression of heat shock proteins HSP70 and HSP27 in primary non-small cell lung carcinomas. An immunohistochemical study. Anticancer Res. 2001. 21:1015–1021.17. Kaur J, Ralhan R. Differential expression of 70-kDa heat shock-protein in human oral tumorigenesis. Int J Cancer. 1995. 63:774–779.
Article18. Cornford PA, Dodson AR, Parsons KF, Desmond AD, Woolfenden A, Fordham M, Neoptolemos JP, Ke Y, Foster CS. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 2000. 60:7099–7105.19. Park CS, Joo IS, Song SY, Kim DS, Bae DS, Lee JH. An immunohistochemical analysis of heat shock protein 70, p53, and estrogen receptor status in carcinoma of the uterine cervix. Gynecol Oncol. 1999. 74:53–60.
Article20. Elpek GO, Karaveli S, Simsek T, Keles N, Aksoy NH. Expression of heat-shock proteins hsp27, hsp70 and hsp90 in malignant epithelial tumour of the ovaries. APMIS. 2003. 111:523–530.21. Ciocca DR, Oesterreich S, Chamness GC, McGuire WL, Fuqua SA. Biological and clinical implications of heat shock protein 27,000 (Hsp27) : a review. J Natl Cancer Inst. 1993. 85:1558–1570.22. King KL, Li AF, Chau GY, Chi CW, Wu CW, Huang CL, Lui WY. Prognostic significance of heat shock protein-27 expression in hepatocellular carcinoma and its relation to histologic grading and survival. Cancer. 2000. 88:2464–2470.
Article23. Harimoto N, Shimada M, Aishima S, Kitagawa D, Itoh S, Tsujita E, Maehara S, Taketomi A, Tanaka S, Shirabe K, Maehara Y. The role of heat shock protein 27 expression in hepatocellular carcinoma in Japan: special reference to the difference between hepatitis B and C. Liver Int. 2004. 24:316–321.
Article24. Chuma M, Sakamoto M, Yamazaki K, Ohta T, Ohki M, Asaka M, Hirohashi S. Expression profiling in multistage hepatocarcinogenesis: identification of HSP70 as a molecular marker of early hepatocellular carcinoma. Hepatology. 2003. 37:198–207.
Article25. Edmondson HA, Steiner PE. Primary carcinoma of the liver: A study of 100 cases among 48,900 necropsies. Cancer. 1954. 7:462–503.26. Yumoto Y, Hanafusa T, Hada H, Morita T, Ooguchi S, Shinji N, Mitani T, Hamaya K, Koide N, Tsuji T. Loss of heterozygosity and analysis of mutation of p53 in hepatocellular carcinoma. J Gastroenterol Hepatol. 1995. 10:179–185.
Article27. Sobin LH, Wittekind C, editors. UICC: TNM classification of malignant tumors. 1997. 5th eds. New York: Wiley-Liss;74–77.28. Esrig D, Elmajian D, Groshen S, Freeman JA, Stein JP, Chen SC, Nichols PW, Skinner DG, Jones PA, Cote RJ. Accumulation of nuclear p53 and tumor progression in bladder cancer. N Engl J Med. 1994. 331:1259–1264.
Article29. Lim SO, Park SJ, Kim W, Park SG, Kim HJ, Kim YI, Sohn TS, Noh JH, Jung G. Proteome analysis of hepatocellular carcinoma. Biochem Biophys Res Commun. 2002. 291:1031–1037.
Article30. Vargas-Roig LM, Fanelli MA, Lopez LA, Gago FE, Tello O, Aznar JC, Ciocca DR. Heat shock proteins and cell proliferation in human breast cancer biopsy samples. Cancer Detect Prev. 1997. 21:441–451.31. Zhao ZG, Shen WL. Heat shock protein 70 antisense oligonucleotide inhibits cell growth and induces apoptosis in human gastric cancer cell line SGC-7901. World J Gastroenterol. 2005. 11:73–78.
Article32. Pinhasi-Kimhi O, Michalovitz D, Ben-Zeev A, Oren M. Specific interaction between the p53 cellular tumour antigen and major heat shock proteins. Nature. 1986. 320:182–184.
Article33. Davidoff AM, Iglehart JD, Marks JR. Immune response to p53 is dependent upon p53/HSP70 complexes in breast cancers. Proc Natl Acad Sci USA. 1992. 89:3439–3442.
Article34. Cui CW, Yang SJ, Liu YP, Liu YF. Interaction between p53 and HSP70 in human hepatocellular carcinoma tissues. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2003. 19:195–196.35. Han J, Yoo HY, Choi BH, Rho HM. Selective transcriptional regulations in the human liver cell by hepatitis B viral X protein. Biochem Biophys Res Commun. 2000. 272:525–530.
Article36. Andrisani OM, Barnabas S. The transcriptional function of the hepatitis B virus X protein and its role in hepatocarcinogenesis. Int J Oncol. 1999. 15:373–379.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Prognostic Impact of Heat Shock Proteins Expression in Patients with Esophageal Cancer: A Meta-Analysis
- The Effect of Yacon (Smallanthus sonchifolius) Extract against Dibutyltin Dichloride-induced Pancreatitis
- Expression of Heat Shock Protein 27 and Alpha B Crystallin in the Retina and Optic Nerve of the Chick Embryo
- Expression of Heat Shock Protein 27 and Apoptosis in Renal Cell Carcinomas
- Role of HSP70 Expression in the Development of Endometrial Adenocarcinoma Correlation of ER, PR, p53, and bcl-2 protein expressions and apoptosis