J Korean Med Sci.
2005 Feb;20(1):36-41. 10.3346/jkms.2005.20.1.36.
Molecular Cytogenetic Analysis of Gene Rearrangements in Childhood Acute Lymphoblastic Leukemia
- Affiliations
-
- 1Department of Laboratory Medicine, Sungkyunkwan University, School of Medicine, Seoul, Korea. sunnyhk@smc.samsung.co.kr
- 2Department of Pediatric Oncology, Sungkyunkwan University, School of Medicine, Seoul, Korea.
- 3Department of Hematology, Sungkyunkwan University, School of Medicine, Seoul, Korea.
- KMID: 1781721
- DOI: http://doi.org/10.3346/jkms.2005.20.1.36
Abstract
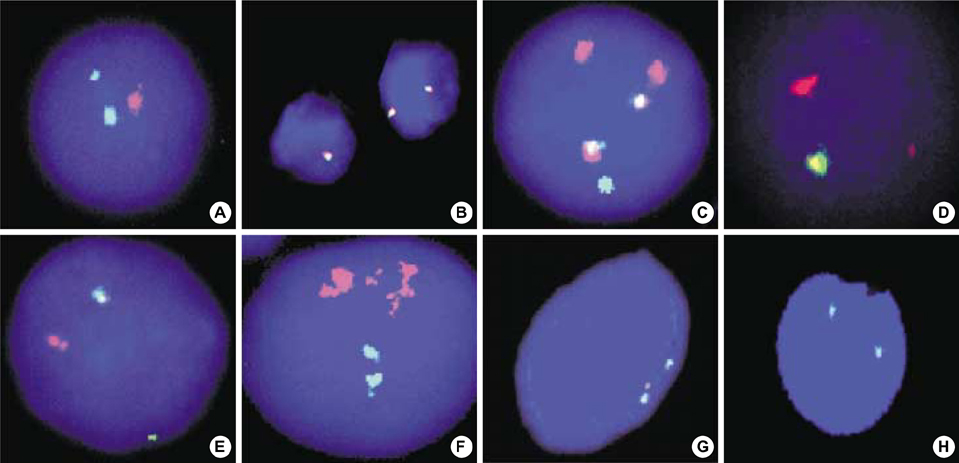
- The aims of this study were to estimate the incidences of BCR/ABL, MLL, TEL/AML1 rearrangements, and p16 deletions in childhood acute lymphoblastic leukemia (ALL), to identify new abnormalities, and to demonstrate the usefulness of interphase fluorescence in situ hybridization (FISH). We performed G-banding analysis and FISH using probes for BCR/ABL, MLL, TEL/AML1 rearrangements, and p16 deletions on 65 childhood ALL patients diagnosed and uniformly treated at a single hospital. Gene rearrangements were identified in 73.8% of the patients using the combination of G-banding and FISH, while the chromosomal abnormalities were identified in 49.2% using G-banding alone. Gene rearrangements were disclosed by FISH in 24 (72.7%) of 33 patients with normal karyotype or no mitotic cell in G-banding. Among the gene rearrangements detected by FISH, the most common gene rearrangement was p16 deletion (20.3%) and the incidences of others were 14.1% for TEL/AML1, 11.3% for MLL, and 1.8% for BCR/ABL translocations. Infrequent or new aberrations such as AML1 amplification, MLL deletion, ABL deletion, and TEL/AML1 fusion with AML1 deletion were also observed. We established the rough incidences of gene rearrangements in childhood ALL, found new abnormalities and demonstrated the diagnostic capability of interphase FISH to identify cryptic chromosome aberrations.
Keyword
MeSH Terms
-
Adolescent
Child
Child, Preschool
*Chromosome Aberrations
Chromosome Banding
DNA-Binding Proteins/*genetics
Female
Fusion Proteins, bcr-abl/*genetics
Gene Deletion
*Gene Rearrangement
Humans
In Situ Hybridization, Fluorescence
Infant
Interphase
Leukemia, Lymphocytic, Acute/*genetics
Male
Oncogene Proteins, Fusion/*genetics
Protein p16/*genetics
Proto-Oncogenes/*genetics
Transcription Factors/*genetics
Treatment Outcome
Figure
Reference
-
1. Ma SK, Wan TS, Chan LC. Cytogenetics and molecular genetics of childhood leukemia. Hematol Oncol. 1999. 17:91–105.
Article2. Kees UR, Burton PR, Lü C, Baker DL. Homozygous deletion of the p16/MTS1 gene in pediatric acute lymphoblastic leukemia is associated with unfavorable clinical outcome. Blood. 1997. 89:4161–4166.
Article3. Heerema NA, Sather HN, Sensel MG, Liu-Mares W, Lange BJ, Bostrom BC, Nachman JB, Steinherz PG, Hutchinson R, Gaynon PS, Arthur DC, Uckun FM. Association of chromosome arm 9p abnormalities with adverse risk in childhood acute lymphoblastic leukemia: a report from the children's cancer group. Blood. 1999. 94:1537–1544.4. Carter TL, Watt PM, Kumar R, Burton PR, Reaman GH, Sather HN, Baker DL, Kees UR. Hemizygous p16INK4A deletion in pediatric acute lymphoblastic leukemia predicts independent risk of relapse. Blood. 2001. 97:572–574.5. Wiemels JL, Ford AM, Van Wering ER, Postma A, Greaves M. Protracted and variable latency of acute lymphoblastic leukemia after TEL-AML1 gene fusion in utero. Blood. 1999. 94:1057–1062.6. Anguita E, Gonzalez FA, Lopez J, Villegas A. TEL/AML1 transcript and p16 gene deletion in a patient with childhood acute lymphoblastic leukemia. Br J Haematol. 1997. 99:240–241.7. Nordgren A, Schoumans J, Söderhäll S, Nordenskjöld M, Blennow E. Interphase fluorescence in situ hybridization and spectral karyotyping reveals hidden genetic aberrations in children with acute lymphoblastic leukemia and a normal banded karyotype. Br J Haematol. 2001. 114:786–793.8. Nordgren A, Heyman M, Sahlén S, Schoumans J, Söderhäll S, Nordenskjöld M, Blennow E. Spectral karyotyping and interphase FISH reveal abnormalities not detected by conventional G-banding. Eur J Haematol. 2002. 68:31–41.9. Garcia-Sanz R, Alaejos I, Orfão A, Rasillo A, Chillón MC, Tabernero MD, Mateos MV, López-Pérez R, González D, Balanzategui A, González M, San Miguel JF. Low frequency of the TEL/AML1 fusion gene in acute lymphoblastic leukemia in Spain. Br J Haematol. 1999. 107:667–669.10. Andreasson P, Höglund M, Békássy AN, Garwicz S, Heldrup J, Mitelman F, Johansson B. Cytogenetic and FISH studies of a single center consecutive series of 152 childhood acute lymphoblastic leukemias. Eur J Haematol. 2000. 65:40–51.
Article11. Mitelman F, editor. ISCN. An international system for human cytogenetic nomenclature. 1995. Basel: Tennessee, S Karger.12. McLean TW, Ringold S, Neuberg D, Stegmaier K, Tantravahi R, Ritz J, Koeffler HP, Takeuchi S, Janssen JW, Seriu T, Bartram CR, Sallan SE, Gilliland DG, Golub TR. TEL/AML-1 dimerizes and is associated with a favorable outcome in childhood acute lymphoblastic leukemia. Blood. 1996. 88:4252–4258.
Article13. Rubnitz JE, Shuster JJ, Land VJ, Link MP, Pullen DJ, Camitta BM, Pui CH, Downing JR, Behm FG. Case-control study suggests a favorable impact of TEL rearrangement in patients with B lineage acute lymphoblastic leukemia treated with antimetabolite based therapy: a Pediatric Oncology Group study. Blood. 1997. 89:1143–1146.14. Borkhardt A, Cazzaniga G, Viehmann S, Valsecchi MG, Ludwig WD, Burci L, Mangioni S, Schrappe M, Riehm H, Lampert F, Basso G, Masera G, Garbott J, Biondi A. Incidence and clinical relevance of TEL/AML1 fusion genes in children with acute lymphoblastic leukemia enrolled in the German and Italian multicenter therapy trials. Blood. 1997. 90:571–577.
Article15. Cavé H, Cacheux V, Raynaud S, Brunie G, Bakkus M, Cochaux P, Preudhomme C, Lai JL, Vilmer E, Grandchamp B. ETV6 is the target of chromosome 12p deletions in t(12;21) childhood acute lymphocytic leukemia. Leukemia. 1997. 11:1459–1464.
Article16. Park KU, She CJ, Shin HY, Ahn HS, Kim CJ, Cho BK, Cho HI, Lee DS. Low incidence of TEL/AML1 fusion and TEL deletion in Korean childhood acute leukemia by extra-signal fluorescence in situ hybridization. Cancer Genet Cytogenet. 2001. 126:73–77.
Article17. Drexler HG. Review of alterations of the cyclin dependent kinase inhibitor INK4 family genes p15, p16, p18 and p19 in human leukemialymphoma cells. Leukemia. 1998. 12:845–859.18. Raynaud S, Cavée H, Baens M, Shurtleff SA, Mathew S, Raimondi S. The 12;21 translocation involving TEL and deletion of the other TEL allele: two frequently associated alterations found in childhood acute lymphoblastic leukemia. Blood. 1996. 87:2891–2899.
Article19. Raynaud SD, Dastugue N, Zoccola D, Shurtleff SA, Mathew S, Raimondi SC. Cytogenetic abnormalities associated with the t(12;21). A collaborative study of 169 children with t(12;21)-positive acute lymphoblastic leukemia. Leukemia. 1999. 13:1325–1330.
Article20. Kempski HM, Sturt NT. The TEL-AML1 fusion accompanied by loss of the untranslocated TEL allele in B-precursor acute lymphoblastic leukemia of childhood. Leukemia Lymphoma. 2000. 40:39–47.21. Kim DH, Moldwin RL, Vignon C, Bohlander SK, Suto Y, Giordano L, Gupta R, Fears S, Nucifora G, Rowley JD, Smith SD. TEL-AML1 translocations with TEL and CDKN2 inactivation in acute lymphoblastic leukemia cell lines. Blood. 1996. 88:785–794.
Article22. Inamdar N, Kumar SA, Banavali SD, Advani S, Magrath I, Bhatia K. Comparative incidence of the rearrangements of TEL/AML1 and ALL1 genes in pediatric precursor B acute lymphoblastic leukemias in India. Int J Oncol. 1998. 13:1319–1322.
Article23. Loncarevic IF, Roltzheim B, Ritterbach J, Viehmann S, Borkhardt A, Lampert F, Harbott J. Trisomy 21 is a recurrent secondary aberration in childhood acute lymphoblastic leukemia with TEL/AML1 gene fusion. Genes Chromosomes Cancer. 1999. 24:272–277.24. Kobayashi H, Espinosa R III, Thirman MJ, Fernald AA, Shannon K, Diaz MO, Le Beau MM, Rowley JD. Do terminal deletions of 11q23 exist? Identification of undetected translocations with fluorescence in situ hybridization. Genes Chrom Cancer. 1993. 7:204–208.
Article25. Harbott J, Mancini M, Verellen-Dumoulin C, Moorman AV, Secker-Walker LM. Hematological malignancies with a deletion of 11q23: cytogenetic and clinical aspects. Leukemia. 1998. 12:823–827.
Article26. Devlin J, Elder PA, Gabra H, Steel CM, Knowles MA. High frequency of chromosome 9 deletion in ovarian cancer: evidence for three tumour suppressor loci. Br J Cancer. 1996. 73:420–423.27. Takita J, Hayashi Y, Kohno T, Yamaguchi N, Hanada R, Yamamoto K, Yokota J. Deletion map of chromosome 9 and p16 (CDKN2A) gene alterations in neuroblastoma. Cancer Res. 1997. 57:907–912.28. Niini T, Kanerva J, Vettenranta K, Saarinen-Pihkalla UM, Knuutila S. AML1 gene amplification: a novel finding in childhood acute lymphoblastic leukemia. Haematologica. 2000. 85:362–366.29. Cin PD, Atkins L, Ford C, Ariyanayagam S, Armstrong SA, George R, Cleary A, Morton CC. Amplification of AML1 in childhood acute lymphoblastic leukemia. Genes Chrom Cancer. 2001. 30:407–409.30. Coniat MB, Khac FN, Daniel MT, Bernard OA, Berger R. Chromosome 21 abnormalities with AML1 amplification in acute lymphoblastic leukemia. Genes Chrom Cancer. 2001. 32:244–249.31. Ma SK, Wan TS, Cheuk AT, Fung LF, Chan GC, Chan SY, Ha SY, Chan LC. Characterization of additional genetic events in childhood acute lymphoblastic leukemia with TEL/AML1 gene fusion: a molecular cytogenetic study. Leukemia. 2001. 15:1442–1447.32. Alvarez Y, Coll MD, Bastida P, Ortega JJ, Caballin MR. AML1 amplification in a child with acute lymphoblastic leukemia. Cancer Genet Cytogenet. 2003. 140:58–61.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Simultaneous Reverse Transcription-Polymerase Chain Reaction for Detection of 7 Gene Rearrangements in Acute Leukemia
- Advances in the Treatment of Childhood Acute Lymphoblastic Leukemia
- Mutational Analysis of CDKN2 (p16-INK4A/MTS1) Gene in Childhood Acute Leukemia
- Meeting Report: 2009 Symposium on Childhood Acute Lymphoblastic Leukemia - Update on the Diagnosis and Treatment for Acute Lymphoblastic Leukemia in Childhood & Adolescence; Seoul; Korea; June 27, 2009
- A Case of Pediatric Acute Lymphoblastic Leukemia with Trisomy 5 as a Sole Chromosomal Anomaly: A Prognostic Significance