Yonsei Med J.
2010 Nov;51(6):954-959. 10.3349/ymj.2010.51.6.954.
Pulsed Electromagnetic Field Stimulates Cellular Proliferation in Human Intervertebral Disc Cells
- Affiliations
-
- 1Department of Orthopaedic Surgery, Yonsei University College of Medicine, Seoul, Korea. shmoon@yuhs.ac
- 2Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1779646
- DOI: http://doi.org/10.3349/ymj.2010.51.6.954
Abstract
- PURPOSE
The purpose of this study is to investigate the mechanism of cellular proliferation of electromagnetic field (EMF) on human intervertebral disc (IVD) cells.
MATERIALS AND METHODS
Human IVD cells were cultured three-dimensionally in alginate beads. EMF was exposed to IVD cells with 650Omega, 1.8 millitesla magnetic flux density, 60 Hz sinusoidal wave. Cultures were divided into a control and EMF group. Cytotoxicity, DNA synthesis and proteoglycan synthesis were measured by MTT assay, [3H]-thymidine, and [35S]-sulfate incorporation. To detect phenotypical expression, reverse transcription-polymerase chain reactions (RT-PCR) were performed for aggrecan, collagen type I, and type II mRNA expression. To assess action mechanism of EMF, IVD cells were exposed to EMF with NG-Monomethyl-L-arginine (NMMA) and acetylsalicylic acid (ASA).
RESULTS
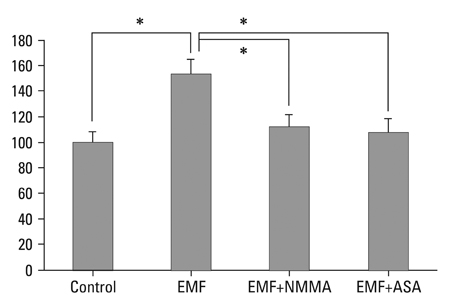
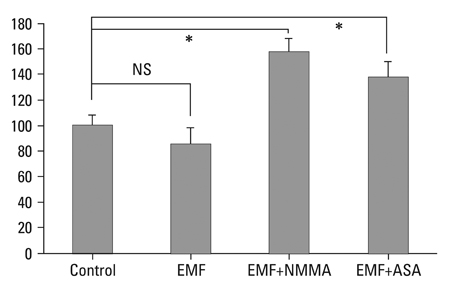
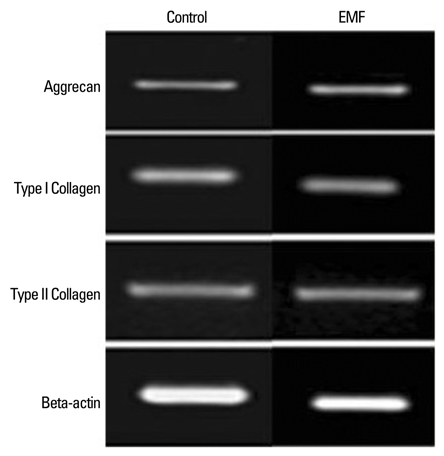
There was no cytotoxicity in IVD cells with the EMF group in MTT assay. Cellular proliferation was observed in the EMF group (p < 0.05). There was no difference in newly synthesized proteoglycan normalized by DNA synthesis between the EMF group and the control. Cultures with EMF showed no significant change in the expression of aggrecan, type I, and type II collagen mRNA compared to the control group. Cultures with NMMA (blocker of nitric oxide) or ASA (blocker of prostaglandin E2) exposed to EMF demonstrated decreased DNA synthesis compared to control cultures without NMMA or ASA (p < 0.05).
CONCLUSION
EMF stimulated DNA synthesis in human IVD cells while no significant effect on proteoglycan synthesis and chondrogenic phenotype expressions. DNA synthesis was partially mediated by nitric oxide and prostaglandin E2. EMF can be utilized to stimulate proliferation of IVD cells, which may provide efficient cell amplification in cell therapy to degenerative disc disease.
MeSH Terms
-
Adult
Aspirin/pharmacology
Cell Proliferation/*radiation effects
Collagen/metabolism
Dinoprostone/metabolism
*Electromagnetic Fields
Enzyme Inhibitors/pharmacology
Female
Humans
Intervertebral Disk/*pathology/radiation effects
Male
Middle Aged
Nitric Oxide/metabolism
Tetrazolium Salts/pharmacology
Thiazoles/pharmacology
omega-N-Methylarginine/pharmacology
Figure
Reference
-
1. Colson DJ, Browett JP, Fiddian NJ, Watson B. Treatment of delayed- and non-union of fractures using pulsed electromagnetic fields. J Biomed Eng. 1988. 10:301–304.
Article2. Foley KT, Mroz TE, Arnold PM, Chandler HC Jr, Dixon RA, Girasole GJ, et al. Randomized, prospective, and controlled clinical trial of pulsed electromagnetic field stimulation for cervical fusion. Spine J. 2008. 8:436–442.3. Holmes GB Jr. Treatment of delayed unions and nonunions of the proximal fifth metatarsal with pulsed electromagnetic fields. Foot Ankle Int. 1994. 15:552–556.
Article4. Borsalino G, Bagnacani M, Bettati E, Fornaciari F, Rocchi R, Uluhogian S, et al. Electrical stimulation of human femoral intertrochanteric osteotomies. Double-blind study. Clin Orthop Relat Res. 1988. (237):256–263.5. Lin HY, Lu KH. Repairing large bone fractures with low frequency electromagnetic fields. J Orthop Res. 2010. 28:265–270.6. Yen-Patton GP, Patton WF, Beer DM, Jacobson BS. Endothelial cell response to pulsed electromagnetic fields: stimulation of growth rate and angiogenesis in vitro. J Cell Physiol. 1988. 134:37–46.
Article7. Schnoke M, Midura RJ. Pulsed electromagnetic fields rapidly modulate intracellular signaling events in osteoblastic cells: comparison to parathyroid hormone and insulin. J Orthop Res. 2007. 25:933–940.
Article8. De Mattei M, Gagliano N, Moscheni C, Dellavia C, Calastrini C, Pellati A, et al. Changes in polyamines, c-myc and c-fos gene expression in osteoblast-like cells exposed to pulsed electromagnetic fields. Bioelectromagnetics. 2005. 26:207–214.9. Fitzsimmons RJ, Ryaby JT, Magee FP, Baylink DJ. IGF-II receptor number is increased in TE-85 osteosarcoma cells by combined magnetic fields. J Bone Miner Res. 1995. 10:812–819.
Article10. Fitzsimmons RJ, Baylink DJ. Growth factors and electromagnetic fields in bone. Clin Plast Surg. 1994. 21:401–406.
Article11. Frazer A, Bunning RA, Thavarajah M, Seid JM, Russell RG. Studies on type II collagen and aggrecan production in human articular chondrocytes in vitro and effects of transforming growth factor-beta and interleukin-1beta. Osteoarthritis Cartilage. 1994. 2:235–245.
Article12. Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg. 2006. 88:Suppl 2. 10–14.
Article13. Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg. 2006. 88:Suppl 2. 21–24.
Article14. Hoogendoorn R, Doulabi BZ, Huang CL, Wuisman PI, Bank RA, Helder MN. Molecular changes in the degenerated goat intervertebral disc. Spine (Phila Pa 1976). 2008. 33:1714–1721.
Article15. Kim H, Lee JU, Moon SH, Kim HC, Kwon UH, Seol NH, et al. Zonal responsiveness of the human intervertebral disc to bone morphogenetic protein-2. Spine (Phila Pa 1976). 2009. 34:1834–1838.
Article16. Kim DJ, Moon SH, Kim H, Kwon UH, Park MS, Han KJ, et al. Bone morphogenetic protein-2 facilitates expression of chondrogenic, not osteogenic, phenotype of human intervertebral disc cells. Spine (Phila Pa 1976). 2003. 28:2679–2684.
Article17. Hiyama A, Mochida J, Iwashina T, Omi H, Watanabe T, Serigano K, et al. Transplantation of mesenchymal stem cells in a canine disc degeneration model. J Orthop Res. 2008. 26:589–600.18. Moon SH, Nishida K, Gilbertson LG, Lee HM, Kim H, Hall RA, et al. Biologic response of human intervertebral disc cells to gene therapy cocktail. Spine (Phila Pa 1976). 2008. 33:1850–1855.
Article19. Nishida K, Kang JD, Gilbertson LG, Moon SH, Suh JK, Vogt MT, et al. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: an in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine (Phila Pa 1976). 1999. 24:2419–2425.20. Yang X, Li X. Nucleus pulposus tissue engineering: a brief review. Eur Spine J. 2009. 18:1564–1572.21. Thompson JP, Pearce RH, Schechter MT, Adams ME, Tsang IK, Bishop PB. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine (Phila Pa 1976). 1990. 15:411–415.
Article22. Chelberg MK, Banks GM, Geiger DF, Oegema TR Jr. Identification of heterogeneous cell populations in normal human intervertebral disc. J Anat. 1995. 186:43–53.23. Maldonado BA, Oegema TR Jr. Initial characterization of the metabolism of intervertebral disc cells encapsulated in microspheres. J Orthop Res. 1992. 10:677–690.24. McLeod KJ, Collazo L. Suppression of a differentiation response in MC-3T3-E1 osteoblast-like cells by sustained, low-level, 30 Hz magnetic-field exposure. Radiat Res. 2000. 153:706–714.
Article25. Ciombor DM, Lester G, Aaron RK, Neame P, Caterson B. Low frequency EMF regulates chondrocyte differentiation and expression of matrix proteins. J Orthop Res. 2002. 20:40–50.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Pulsed Electromagnetic Field in Human Intervertebral Disc Cell
- The effects of static magnetic field and pulsed electromagnetic field on alkaline phosphatase and dna synthetic activity of ME3t3-E1 cells
- Nitric Oxide Production of Rat Osteoblast Cells by Pulsed Electromagnetic Field Radiation
- The Effect of Lactic Acid Concentration on Cell Morphology and Phenotype in Cultured Intervertebral Disc Cell of Rabbit
- Biochemical Factors of Intervertebral Disc Degeneration: Implications for Disc Regeneration