Yonsei Med J.
2010 Nov;51(6):808-822. 10.3349/ymj.2010.51.6.808.
Protease and Protease-Activated Receptor-2 Signaling in the Pathogenesis of Atopic Dermatitis
- Affiliations
-
- 1Department of Dermatology, Yonsei University College of Medicine, Seoul, Korea. ydshderm@yuhs.ac
- 2Human Barrier Research Institute, Yonsei University College of Medicine, Seoul, Korea.
- 3Research Division, NeoPharm Co., Ltd., Daejeon, Korea.
- KMID: 1779625
- DOI: http://doi.org/10.3349/ymj.2010.51.6.808
Abstract
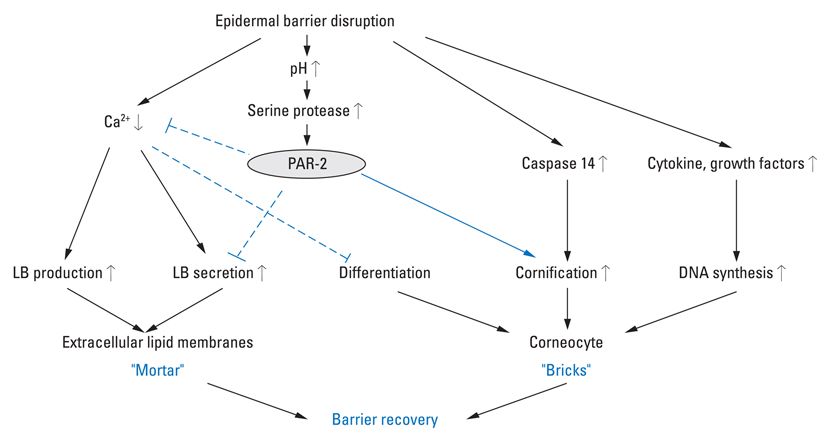
- Proteases in the skin are essential to epidermal permeability barrier homeostasis. In addition to their direct proteolytic effects, certain proteases signal to cells by activating protease-activated receptors (PARs), the G-protein-coupled receptors. The expression of functional PAR-2 on human skin and its role in inflammation, pruritus, and skin barrier homeostasis have been demonstrated. Atopic dermatitis (AD) is a multifactorial inflammatory skin disease characterized by genetic barrier defects and allergic inflammation, which is sustained by gene-environmental interactions. Recent studies have revealed aberrant expression and activation of serine proteases and PAR-2 in the lesional skin of AD patients. The imbalance between proteases and protease inhibitors associated with genetic defects in the protease/protease inhibitor encoding genes, increase in skin surface pH, and exposure to proteolytically active allergens contribute to this aberrant protease/PAR-2 signaling in AD. The increased protease activity in AD leads to abnormal desquamation, degradation of lipid-processing enzymes and antimicrobial peptides, and activation of primary cytokines, thereby leading to permeability barrier dysfunction, inflammation, and defects in the antimicrobial barrier. Moreover, up-regulated proteases stimulate PAR-2 in lesional skin of AD and lead to the production of cytokines and chemokines involved in inflammation and immune responses, itching sensation, and sustained epidermal barrier perturbation with easier allergen penetration. In addition, PAR-2 is an important sensor for exogenous danger molecules, such as exogenous proteases from various allergens, and plays an important role in AD pathogenesis. Together, these findings suggest that protease activity or PAR-2 may be a future target for therapeutic intervention for the treatment of AD.
MeSH Terms
-
Anti-Infective Agents/pharmacology
Dermatitis, Atopic/*enzymology
Endopeptidases/metabolism
Homeostasis
Humans
Hydrogen-Ion Concentration
Inflammation
Models, Biological
Models, Genetic
Peptide Hydrolases/*metabolism
Receptor, PAR-2/*metabolism
Serine Proteases/metabolism
Signal Transduction
Skin/enzymology/pathology
Treatment Outcome
Figure
Cited by 1 articles
-
Physical and biochemical characteristics of allergens
Kyoung Yong Jeong
Allergy Asthma Respir Dis. 2016;4(3):157-166. doi: 10.4168/aard.2016.4.3.157.
Reference
-
1. Richard I. The genetic and molecular bases of monogenic disorders affecting proteolytic systems. J Med Genet. 2005. 42:529–539.
Article2. Ramachandran R, Hollenberg MD. Proteinases and signalling: pathophysiological and therapeutic implications via PARs and more. Br J Pharmacol. 2008. 153:Suppl 1. S263–S282.3. Brattsand M, Egelrud T. Purification, molecular cloning, and expression of a human stratum corneum trypsin-like serine protease with possible function in desquamation. J Biol Chem. 1999. 274:30033–30040.
Article4. Hansson L, Strömqvist M, Bäckman A, Wallbrandt P, Carlstein A, Egelrud T. Cloning, expression, and characterization of stratum corneum chymotryptic enzyme. A skin-specific human serine proteinase. J Biol Chem. 1994. 269:19420–19426.
Article5. Bernard D, Méhul B, Thomas-Collignon A, Simonetti L, Remy V, Bernard MA, et al. Analysis of proteins with caseinolytic activity in a human stratum corneum extract revealed a yet unidentified cysteine protease and identified the so-called "stratum corneum thiol protease" as cathepsin l2. J Invest Dermatol. 2003. 120:592–600.
Article6. Horikoshi T, Arany I, Rajaraman S, Chen SH, Brysk H, Lei G, et al. Isoforms of cathepsin D and human epidermal differentiation. Biochimie. 1998. 80:605–612.
Article7. Hachem JP, Houben E, Crumrine D, Man MQ, Schurer N, Roelandt T, et al. Serine protease signaling of epidermal permeability barrier homeostasis. J Invest Dermatol. 2006. 126:2074–2086.
Article8. Rattenholl A, Steinhoff M. Role of proteinase-activated receptors in cutaneous biology and disease. Drug Dev Res. 2003. 59:408–416.
Article9. Hansen KK, Oikonomopoulou K, Baruch A, Ramachandran R, Beck P, Diamandis EP, et al. Proteinases as hormones: targets and mechanisms for proteolytic signaling. Biol Chem. 2008. 389:971–982.
Article10. Rattenholl A, Steinhoff M. Proteinase-activated receptor-2 in the skin: receptor expression, activation and function during health and disease. Drug News Perspect. 2008. 21:369–381.
Article11. Demerjian M, Hachem JP, Tschachler E, Denecker G, Declercq W, Vandenabeele P, et al. Acute modulations in permeability barrier function regulate epidermal cornification: role of caspase-14 and the protease-activated receptor type 2. Am J Pathol. 2008. 172:86–97.12. Jeong SK, Kim HJ, Youm JK, Ahn SK, Choi EH, Sohn MH, et al. Mite and cockroach allergens activate protease-activated receptor 2 and delay epidermal permeability barrier recovery. J Invest Dermatol. 2008. 128:1930–1939.
Article13. Eissa A, Diamandis EP. Human tissue kallikreins as promiscuous modulators of homeostatic skin barrier functions. Biol Chem. 2008. 389:669–680.
Article14. Stefansson K, Brattsand M, Roosterman D, Kempkes C, Bocheva G, Steinhoff M, et al. Activation of proteinase-activated receptor-2 by human kallikrein-related peptidases. J Invest Dermatol. 2008. 128:18–25.
Article15. Descargues P, Deraison C, Prost C, Fraitag S, Mazereeuw-Hautier J, D'Alessio M, et al. Corneodesmosomal cadherins are preferential targets of stratum corneum trypsin- and chymotrypsin-like hyperactivity in Netherton syndrome. J Invest Dermatol. 2006. 126:1622–1632.
Article16. Komatsu N, Suga Y, Saijoh K, Liu AC, Khan S, Mizuno Y, et al. Elevated human tissue kallikrein levels in the stratum corneum and serum of peeling skin syndrome-type B patients suggests an over-desquamation of corneocytes. J Invest Dermatol. 2006. 126:2338–2342.
Article17. Komatsu N, Saijoh K, Kuk C, Liu AC, Khan S, Shirasaki F, et al. Human tissue kallikrein expression in the stratum corneum and serum of atopic dermatitis patients. Exp Dermatol. 2007. 16:513–519.
Article18. Komatsu N, Saijoh K, Kuk C, Shirasaki F, Takehara K, Diamandis EP. Aberrant human tissue kallikrein levels in the stratum corneum and serum of patients with psoriasis: dependence on phenotype, severity and therapy. Br J Dermatol. 2007. 156:875–883.
Article19. Lundwall A, Brattsand M. Kallikrein-related peptidases. Cell Mol Life Sci. 2008. 65:2019–2038.
Article20. Caubet C, Jonca N, Brattsand M, Guerrin M, Bernard D, Schmidt R, et al. Degradation of corneodesmosome proteins by two serine proteases of the kallikrein family, SCTE/KLK5/hK5 and SCCE/KLK7/hK7. J Invest Dermatol. 2004. 122:1235–1244.
Article21. Hachem JP, Man MQ, Crumrine D, Uchida Y, Brown BE, Rogiers V, et al. Sustained serine proteases activity by prolonged increase in pH leads to degradation of lipid processing enzymes and profound alterations of barrier function and stratum corneum integrity. J Invest Dermatol. 2005. 125:510–520.
Article22. Yamasaki K, Schauber J, Coda A, Lin H, Dorschner RA, Schechter NM, et al. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006. 20:2068–2080.23. Ekholm IE, Brattsand M, Egelrud T. Stratum corneum tryptic enzyme in normal epidermis: a missing link in the desquamation process? J Invest Dermatol. 2000. 114:56–63.24. Brattsand M, Stefansson K, Lundh C, Haasum Y, Egelrud T. A proteolytic cascade of kallikreins in the stratum corneum. J Invest Dermatol. 2005. 124:198–203.
Article25. Elias PM, Cullander C, Mauro T, Rassner U, Kömüves L, Brown BE, et al. The secretory granular cell: the outermost granular cell as a specialized secretory cell. J Investig Dermatol Symp Proc. 1998. 3:87–100.
Article26. Yoon H, Laxmikanthan G, Lee J, Blaber SI, Rodriguez A, Kogot JM, et al. Activation profiles and regulatory cascades of the human kallikrein-related peptidases. J Biol Chem. 2007. 282:31852–31864.
Article27. Oikonomopoulou K, Hansen KK, Saifeddine M, Tea I, Blaber M, Blaber SI, et al. Proteinase-activated receptors, targets for kallikrein signaling. J Biol Chem. 2006. 281:32095–32112.
Article28. Komatsu N, Saijoh K, Toyama T, Ohka R, Otsuki N, Hussack G, et al. Multiple tissue kallikrein mRNA and protein expression in normal skin and skin diseases. Br J Dermatol. 2005. 153:274–281.
Article29. Kishibe M, Bando Y, Terayama R, Namikawa K, Takahashi H, Hashimoto Y, et al. Kallikrein 8 is involved in skin desquamation in cooperation with other kallikreins. J Biol Chem. 2007. 282:5834–5841.30. Morizane S, Yamasaki K, Kabigting FD, Gallo RL. Kallikrein expression and cathelicidin processing are independently controlled in keratinocytes by calcium, vitamin D(3), and retinoic acid. J Invest Dermatol. 2010. 130:1297–1306.
Article31. Ovaere P, Lippens S, Vandenabeele P, Declercq W. The emerging roles of serine protease cascades in the epidermis. Trends Biochem Sci. 2009. 34:453–463.
Article32. List K, Szabo R, Wertz PW, Segre J, Haudenschild CC, Kim SY, et al. Loss of proteolytically processed filaggrin caused by epidermal deletion of Matriptase/MT-SP1. J Cell Biol. 2003. 163:901–910.33. Mitsudo K, Jayakumar A, Henderson Y, Frederick MJ, Kang Y, Wang M, et al. Inhibition of serine proteinases plasmin, trypsin, subtilisin A, cathepsin G, and elastase by LEKTI: a kinetic analysis. Biochemistry. 2003. 42:3874–3881.
Article34. Roelandt T, Thys B, Heughebaert C, De Vroede A, De Paepe K, Roseeuw D, et al. LEKTI-1 in sickness and in health. Int J Cosmet Sci. 2009. 31:247–254.35. Meyer-Hoffert U, Wu Z, Schröder JM. Identification of lympho-epithelial Kazal-type inhibitor 2 in human skin as a kallikrein-related peptidase 5-specific protease inhibitor. PLoS One. 2009. 4:e4372.36. Galliano MF, Toulza E, Gallinaro H, Jonca N, Ishida-Yamamoto A, Serre G, et al. A novel protease inhibitor of the alpha2-macroglobulin family expressed in the human epidermis. J Biol Chem. 2006. 281:5780–5789.
Article37. Oberst MD, Chen LY, Kiyomiya K, Williams CA, Lee MS, Johnson MD, et al. HAI-1 regulates activation and expression of matriptase, a membrane-bound serine protease. Am J Physiol Cell Physiol. 2005. 289:C462–C470.
Article38. Oberst MD, Williams CA, Dickson RB, Johnson MD, Lin CY. The activation of matriptase requires its noncatalytic domains, serine protease domain, and its cognate inhibitor. J Biol Chem. 2003. 278:26773–26779.39. Kawabata A. PAR-2: structure, function and relevance to human diseases of the gastric mucosa. Expert Rev Mol Med. 2002. 4:1–17.
Article40. Steinhoff M, Corvera CU, Thoma MS, Kong W, McAlpine BE, Caughey GH, et al. Proteinase-activated receptor-2 in human skin: tissue distribution and activation of keratinocytes by mast cell tryptase. Exp Dermatol. 1999. 8:282–294.41. Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, et al. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci. 2003. 23:6176–6180.
Article42. Scott G, Deng A, Rodriguez-Burford C, Seiberg M, Han R, Babiarz L, et al. Protease-activated receptor 2, a receptor involved in melanosome transfer, is upregulated in human skin by ultraviolet irradiation. J Invest Dermatol. 2001. 117:1412–1420.43. Déry O, Thoma MS, Wong H, Grady EF, Bunnett NW. Trafficking of proteinase-activated receptor-2 and beta-arrestin-1 tagged with green fluorescent protein. beta-Arrestin-dependent endocytosis of a proteinase receptor. J Biol Chem. 1999. 274:18524–18535.44. Santulli RJ, Derian CK, Darrow AL, Tomko KA, Eckardt AJ, Seiberg M, et al. Evidence for the presence of a protease-activated receptor distinct from the thrombin receptor in human keratinocytes. Proc Natl Acad Sci U S A. 1995. 92:9151–9155.45. Derian CK, Eckardt AJ, Andrade-Gordon P. Differential regulation of human keratinocyte growth and differentiation by a novel family of protease-activated receptors. Cell Growth Differ. 1997. 8:743–749.46. Scott IR, Harding CR, Barrett JG. Histidine-rich protein of the keratohyalin granules. Source of the free amino acids, urocanic acid and pyrrolidone carboxylic acid in the stratum corneum. Biochim Biophys Acta. 1982. 719:110–117.47. McGrath JA, Uitto J. The filaggrin story: novel insights into skin-barrier function and disease. Trends Mol Med. 2008. 14:20–27.
Article48. Denecker G, Hoste E, Gilbert B, Hochepied T, Ovaere P, Lippens S, et al. Caspase-14 protects against epidermal UVB photodamage and water loss. Nat Cell Biol. 2007. 9:666–674.
Article49. Resing KA, Thulin C, Whiting K, al-Alawi N, Mostad S. Characterization of profilaggrin endoproteinase 1. A regulated cytoplasmic endoproteinase of epidermis. J Biol Chem. 1995. 270:28193–28198.50. Yamazaki M, Ishidoh K, Suga Y, Saido TC, Kawashima S, Suzuki K, et al. Cytoplasmic processing of human profilaggrin by active mu-calpain. Biochem Biophys Res Commun. 1997. 235:652–656.51. Pearton DJ, Nirunsuksiri W, Rehemtulla A, Lewis SP, Presland RB, Dale BA. Proprotein convertase expression and localization in epidermis: evidence for multiple roles and substrates. Exp Dermatol. 2001. 10:193–203.
Article52. Leyvraz C, Charles RP, Rubera I, Guitard M, Rotman S, Breiden B, et al. The epidermal barrier function is dependent on the serine protease CAP1/Prss8. J Cell Biol. 2005. 170:487–496.
Article53. Netzel-Arnett S, Currie BM, Szabo R, Lin CY, Chen LM, Chai KX, et al. Evidence for a matriptase-prostasin proteolytic cascade regulating terminal epidermal differentiation. J Biol Chem. 2006. 281:32941–32945.
Article54. Borgoño CA, Michael IP, Komatsu N, Jayakumar A, Kapadia R, Clayman GL, et al. A potential role for multiple tissue kallikrein serine proteases in epidermal desquamation. J Biol Chem. 2007. 282:3640–3652.
Article55. Deraison C, Bonnart C, Lopez F, Besson C, Robinson R, Jayakumar A, et al. LEKTI fragments specifically inhibit KLK5, KLK7, and KLK14 and control desquamation through a pH-dependent interaction. Mol Biol Cell. 2007. 18:3607–3619.
Article56. Zeeuwen PL, Cheng T, Schalkwijk J. The biology of cystatin M/E and its cognate target proteases. J Invest Dermatol. 2009. 129:1327–1338.
Article57. Cheng T, Hitomi K, van Vlijmen-Willems IM, de Jongh GJ, Yamamoto K, Nishi K, et al. Cystatin M/E is a high affinity inhibitor of cathepsin V and cathepsin L by a reactive site that is distinct from the legumain-binding site. A novel clue for the role of cystatin M/E in epidermal cornification. J Biol Chem. 2006. 281:15893–15899.
Article58. Cheng T, Tjabringa GS, van Vlijmen-Willems IM, Hitomi K, van Erp PE, Schalkwijk J, et al. The cystatin M/E-controlled pathway of skin barrier formation: expression of its key components in psoriasis and atopic dermatitis. Br J Dermatol. 2009. 161:253–264.
Article59. Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009. 30:131–141.
Article60. Aberg KM, Man MQ, Gallo RL, Ganz T, Crumrine D, Brown BE, et al. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J Invest Dermatol. 2008. 128:917–925.
Article61. Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007. 13:975–980.
Article62. Seeliger S, Derian CK, Vergnolle N, Bunnett NW, Nawroth R, Schmelz M, et al. Proinflammatory role of proteinase-activated receptor-2 in humans and mice during cutaneous inflammation in vivo. FASEB J. 2003. 17:1871–1885.63. Iwakiri K, Ghazizadeh M, Jin E, Fujiwara M, Takemura T, Takezaki S, et al. Human airway trypsin-like protease induces PAR-2-mediated IL-8 release in psoriasis vulgaris. J Invest Dermatol. 2004. 122:937–944.
Article64. Feingold KR, Schmuth M, Elias PM. The regulation of permeability barrier homeostasis. J Invest Dermatol. 2007. 127:1574–1576.
Article65. Denda M, Kitamura K, Elias PM, Feingold KR. trans-4-(Aminomethyl)cyclohexane carboxylic acid (T-AMCHA), an anti-fibrinolytic agent, accelerates barrier recovery and prevents the epidermal hyperplasia induced by epidermal injury in hairless mice and humans. J Invest Dermatol. 1997. 109:84–90.
Article66. Steinhoff M, Buddenkotte J, Shpacovitch V, Rattenholl A, Moormann C, Vergnolle N, et al. Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocr Rev. 2005. 26:1–43.
Article67. Buddenkotte J, Stroh C, Engels IH, Moormann C, Shpacovitch VM, Seeliger S, et al. Agonists of proteinase-activated receptor-2 stimulate upregulation of intercellular cell adhesion molecule-1 in primary human keratinocytes via activation of NF-kappa B. J Invest Dermatol. 2005. 124:38–45.
Article68. Wakita H, Furukawa F, Takigawa M. Thrombin and trypsin induce granulocyte-macrophage colony-stimulating factor and interleukin-6 gene expression in cultured normal human keratinocytes. Proc Assoc Am Physicians. 1997. 109:190–207.69. Hou L, Kapas S, Cruchley AT, Macey MG, Harriott P, Chinni C, et al. Immunolocalization of protease-activated receptor-2 in skin: receptor activation stimulates interleukin-8 secretion by keratinocytes in vitro. Immunology. 1998. 94:356–362.70. Kawagoe J, Takizawa T, Matsumoto J, Tamiya M, Meek SE, Smith AJ, et al. Effect of protease-activated receptor-2 deficiency on allergic dermatitis in the mouse ear. Jpn J Pharmacol. 2002. 88:77–84.71. Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt Rde W, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007. 25:193–219.
Article72. Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, Brewer A, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005. 202:541–549.
Article73. Ziegler SF, Liu YJ. Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. Nat Immunol. 2006. 7:709–714.
Article74. Briot A, Deraison C, Lacroix M, Bonnart C, Robin A, Besson C, et al. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med. 2009. 206:1135–1147.
Article75. Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000. 6:151–158.
Article76. Paus R, Schmelz M, Bíró T, Steinhoff M. Frontiers in pruritus research: scratching the brain for more effective itch therapy. J Clin Invest. 2006. 116:1174–1186.
Article77. Steinhoff M, Bienenstock J, Schmelz M, Maurer M, Wei E, Bíró T. Neurophysiological, neuroimmunological, and neuroendocrine basis of pruritus. J Invest Dermatol. 2006. 126:1705–1718.
Article78. Costa R, Marotta DM, Manjavachi MN, Fernandes ES, Lima-Garcia JF, Paszcuk AF, et al. Evidence for the role of neurogenic inflammation components in trypsin-elicited scratching behaviour in mice. Br J Pharmacol. 2008. 154:1094–1103.
Article79. Steinhoff M, Stander S, Seeliger S, Ansel JC, Schmelz M, Luger T. Modern aspects of cutaneous neurogenic inflammation. Arch Dermatol. 2003. 139:1479–1488.
Article80. Komatsu N, Tsai B, Sidiropoulos M, Saijoh K, Levesque MA, Takehara K, et al. Quantification of eight tissue kallikreins in the stratum corneum and sweat. J Invest Dermatol. 2006. 126:925–929.
Article81. Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, et al. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci. 2004. 24:4300–4312.
Article82. Vasilopoulos Y, Cork MJ, Murphy R, Williams HC, Robinson DA, Duff GW, et al. Genetic association between an AACC insertion in the 3'UTR of the stratum corneum chymotryptic enzyme gene and atopic dermatitis. J Invest Dermatol. 2004. 123:62–66.
Article83. Hansson L, Bäckman A, Ny A, Edlund M, Ekholm E, Ekstrand Hammarström B, et al. Epidermal overexpression of stratum corneum chymotryptic enzyme in mice: a model for chronic itchy dermatitis. J Invest Dermatol. 2002. 118:444–449.
Article84. Walley AJ, Chavanas S, Moffatt MF, Esnouf RM, Ubhi B, Lawrence R, et al. Gene polymorphism in Netherton and common atopic disease. Nat Genet. 2001. 29:175–178.
Article85. Mägert HJ, Ständker L, Kreutzmann P, Zucht HD, Reinecke M, Sommerhoff CP, et al. LEKTI, a novel 15-domain type of human serine proteinase inhibitor. J Biol Chem. 1999. 274:21499–21502.
Article86. Hachem JP, Wagberg F, Schmuth M, Crumrine D, Lissens W, Jayakumar A, et al. Serine protease activity and residual LEKTI expression determine phenotype in Netherton syndrome. J Invest Dermatol. 2006. 126:1609–1621.
Article87. Descargues P, Deraison C, Bonnart C, Kreft M, Kishibe M, Ishida-Yamamoto A, et al. Spink5-deficient mice mimic Netherton syndrome through degradation of desmoglein 1 by epidermal protease hyperactivity. Nat Genet. 2005. 37:56–65.
Article88. Bonnart C, Deraison C, Lacroix M, Uchida Y, Besson C, Robin A, et al. Elastase 2 is expressed in human and mouse epidermis and impairs skin barrier function in Netherton syndrome through filaggrin and lipid misprocessing. J Clin Invest. 2010. 120:871–882.
Article89. Kato A, Fukai K, Oiso N, Hosomi N, Murakami T, Ishii M. Association of SPINK5 gene polymorphisms with atopic dermatitis in the Japanese population. Br J Dermatol. 2003. 148:665–669.
Article90. Vasilopoulos Y, Cork MJ, Teare D, Marinou I, Ward SJ, Duff GW, et al. A nonsynonymous substitution of cystatin A, a cysteine protease inhibitor of house dust mite protease, leads to decreased mRNA stability and shows a significant association with atopic dermatitis. Allergy. 2007. 62:514–519.
Article91. Mauro T. Eilas P, Feingold K, editors. SC pH: measurement, origins, and functions. Skin Barrier. 2006. New York: Taylor & Francis Group;223–230.92. Fluhr JW, Behne MJ, Brown BE, Moskowitz DG, Selden C, Mao-Qiang M, et al. Stratum corneum acidification in neonatal skin: secretory phospholipase A2 and the sodium/hydrogen antiporter-1 acidify neonatal rat stratum corneum. J Invest Dermatol. 2004. 122:320–329.
Article93. Schmid-Wendtner MH, Korting HC. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol Physiol. 2006. 19:296–302.
Article94. Rippke F, Schreiner V, Doering T, Maibach HI. Stratum corneum pH in atopic dermatitis: impact on skin barrier function and colonization with Staphylococcus Aureus. Am J Clin Dermatol. 2004. 5:217–223.95. Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006. 38:441–446.
Article96. O'Regan GM, Sandilands A, McLean WH, Irvine AD. Filaggrin in atopic dermatitis. J Allergy Clin Immunol. 2008. 122:689–693.97. Fluhr JW, Elias PM, Man MQ, Hupe M, Selden C, Sundberg JP, et al. Is the filaggrin-histidine-urocanic acid pathway essential for stratum corneum acidification? J Invest Dermatol. 2010. 130:2141–2144.
Article98. Roelandt T, Heughebaert C, Hachem JP. Proteolytically active allergens cause barrier breakdown. J Invest Dermatol. 2008. 128:1878–1880.
Article99. Kato T, Takai T, Fujimura T, Matsuoka H, Ogawa T, Murayama K, et al. Mite serine protease activates protease-activated receptor-2 and induces cytokine release in human keratinocytes. Allergy. 2009. 64:1366–1374.
Article100. Hirasawa Y, Takai T, Nakamura T, Mitsuishi K, Gunawan H, Suto H, et al. Staphylococcus aureus extracellular protease causes epidermal barrier dysfunction. J Invest Dermatol. 2010. 130:614–617.
Article101. Ohnemus U, Kohrmeyer K, Houdek P, Rohde H, Wladykowski E, Vidal S, et al. Regulation of epidermal tight-junctions (TJ) during infection with exfoliative toxin-negative Staphylococcus strains. J Invest Dermatol. 2008. 128:906–916.
Article102. Wan H, Winton HL, Soeller C, Tovey ER, Gruenert DC, Thompson PJ, et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999. 104:123–133.
Article103. Runswick S, Mitchell T, Davies P, Robinson C, Garrod DR. Pollen proteolytic enzymes degrade tight junctions. Respirology. 2007. 12:834–842.
Article104. Kawabata A, Kawao N. Physiology and pathophysiology of proteinase-activated receptors (PARs): PARs in the respiratory system: cellular signaling and physiological/pathological roles. J Pharmacol Sci. 2005. 97:20–24.105. Ferrell WR, Lockhart JC, Kelso EB, Dunning L, Plevin R, Meek SE, et al. Essential role for proteinase-activated receptor-2 in arthritis. J Clin Invest. 2003. 111:35–41.106. Hyun E, Andrade-Gordon P, Steinhoff M, Vergnolle N. Protease-activated receptor-2 activation: a major actor in intestinal inflammation. Gut. 2008. 57:1222–1229.107. Kim H, Jeong S, Jeong M, Ahn J, Moon S, Lee S. The relationship of PAR2 and pruritus in end stage renal disease patients and the clinical effectiveness of soybean extracts containing moisturizer on epidermal permeability barrier in end stage renal disease patients. J Invest Dermatol. 2010. 130:S56.108. Akiyama T, Carstens MI, Carstens E. Enhanced scratching evoked by PAR-2 agonist and 5-HT but not histamine in a mouse model of chronic dry skin itch. Pain. 2010. 08. 13. (Epub ahead of print).
Article109. Kelso EB, Lockhart JC, Hembrough T, Dunning L, Plevin R, Hollenberg MD, et al. Therapeutic promise of proteinase-activated receptor-2 antagonism in joint inflammation. J Pharmacol Exp Ther. 2006. 316:1017–1024.110. Kelso EB, Ferrell WR, Lockhart JC, Elias-Jones I, Hembrough T, Dunning L, et al. Expression and proinflammatory role of proteinase-activated receptor 2 in rheumatoid synovium: ex vivo studies using a novel proteinase-activated receptor 2 antagonist. Arthritis Rheum. 2007. 56:765–771.111. Kanke T, Kabeya M, Kubo S, Kondo S, Yasuoka K, Tagashira J, et al. Novel antagonists for proteinase-activated receptor 2: inhibition of cellular and vascular responses in vitro and in vivo. Br J Pharmacol. 2009. 158:361–371.112. Goh FG, Ng PY, Nilsson M, Kanke T, Plevin R. Dual effect of the novel peptide antagonist K-14585 on proteinase-activated receptor-2-mediated signalling. Br J Pharmacol. 2009. 158:1695–1704.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Improvement of Atopic Dermatitis by a Topical Cream Containing a Protease-Activated Receptor-2 (PAR-2) Inhibitor 'Pal-KTTKS Peptide'
- Skin Barrier Recovery by Protease-Activated Receptor-2 Antagonist Lobaric Acid
- Atopic Dermatitis and Epidermal Barrier
- Activation of Eosinophils with Airborne Fungi
- Protease from Airborn Fungi Induce Activation of Nasal Epithelial Cells