Yonsei Med J.
2010 Jan;51(1):104-110. 10.3349/ymj.2010.51.1.104.
Comparisons of Three Automated Systems for Genomic DNA Extraction in a Clinical Diagnostic Laboratory
- Affiliations
-
- 1Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea. kimhs54@yuhs.ac
- KMID: 1779614
- DOI: http://doi.org/10.3349/ymj.2010.51.1.104
Abstract
- PURPOSE
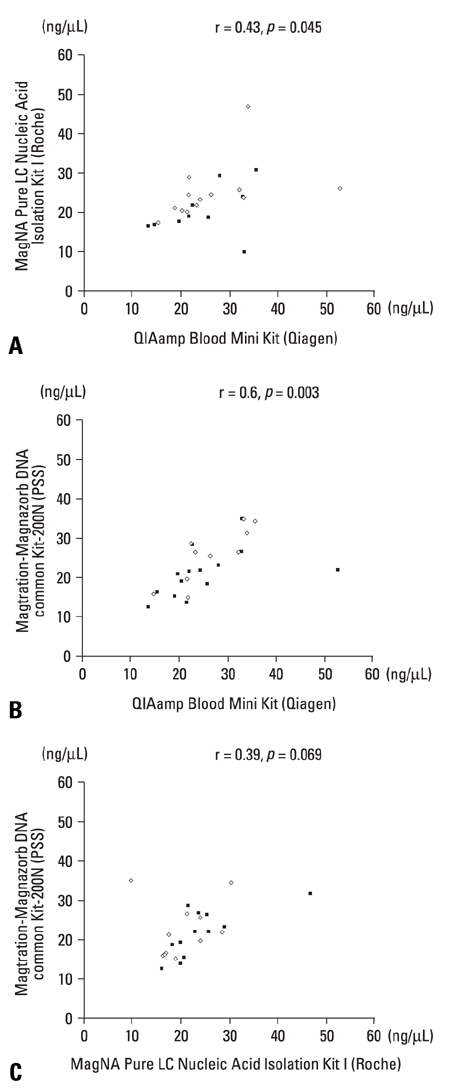
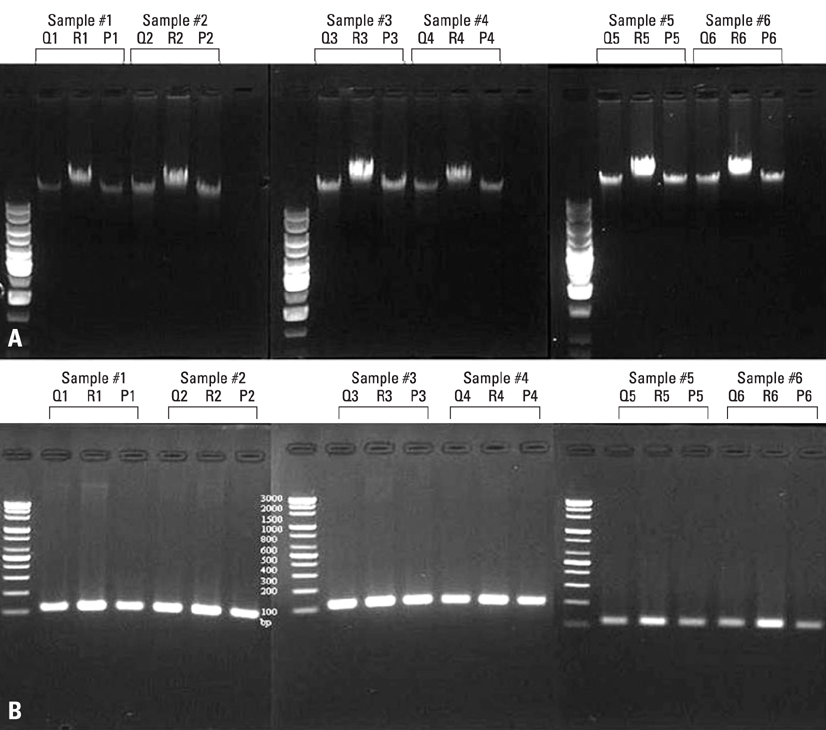
The extraction of nucleic acid is initially a limiting step for successful molecular-based diagnostic workup. This study aims to compare the effectiveness of three automated DNA extraction systems for clinical laboratory use. MATERIALS AND METHODS: Venous blood samples from 22 healthy volunteers were analyzed using QIAamp(R) Blood Mini Kit (Qiagen), MagNA Pure LC Nucleic Acid Isolation Kit I (Roche), and Magtration-Magnazorb DNA common kit-200N (PSS). The concentration of extracted DNAs was measured by NanoDrop ND-1000 (PeqLab). Also, extracted DNAs were confirmed by applying in direct agarose gel electrophoresis and were amplified by polymerase chain reaction (PCR) for human beta-globin gene. RESULTS: The corrected concentrations of extracted DNAs were 25.42 +/- 8.82 ng/microLiter (13.49-52.85 ng/microLiter) by QIAamp(R) Blood Mini Kit (Qiagen), and 22.65 +/- 14.49 ng/microLiter (19.18-93.39 ng/microLiter) by MagNA Pure LC Nucleic Acid Isolation Kit I, and 22.35 +/- 6.47 ng/microLiter (12.57-35.08 ng/microLiter) by Magtration-Magnazorb DNA common kit-200N (PSS). No statistically significant difference was noticed among the three commercial kits (p > 0.05). Only the mean value of DNA purity through PSS was slightly lower than others. All the extracted DNAs were successfully identified in direct agarose gel electrophoresis. And all the product of beta-globin gene PCR showed a reproducible pattern of bands. CONCLUSION: The effectiveness of the three automated extraction systems is of an equivalent level and good enough to produce reasonable results. Each laboratory could select the automated system according to its clinical and laboratory conditions.
MeSH Terms
Figure
Reference
-
1. Bogner PN, Killeen AA. Coleman WB, Tsongalis GJ, editors. Extraction of nucleic acids. Molecular diagnostics for the clinical laboratorian. 2006. 2nd ed. New Jersey: Humana Press;25–30.2. Edvinsson B, Jalal S, Nord CE, Pedersen BS, Evengård B. DNA extraction and PCR assays for detection of Toxoplasma gondii. APMIS. 2004. 112:342–348.3. Riemann K, Adamzik M, Frauenrath S, Egensperger R, Schmid KW, Brockmeyer NH, et al. Comparison of manual and automated nucleic acid extraction from whole-blood samples. J Clin Labo Anal. 2007. 21:244–248.
Article4. Gustafson S, Proper JA, Bowie EJ, Sommer SS. Parameters affecting the yield of DNA from human blood. Anal Biochem. 1987. 165:294–299.
Article5. Garg UC, Hanson NQ, Tsai MY, Eckfeldt JH. Simple and rapid method for extraction of DNA from fresh and cryopreserved clotted human blood. Clin Chem. 1996. 42:647–648.
Article6. Saltman DL, Hunger SP, Turner GE. Gosden JR, editor. Molecular analysis of chromosome aberrations in Hematological Malignancies. Chromosome Analysis Protocols. 1994. New Jersey: Humana Press;437–448.7. Mahuku GS. A simple extraction method suitable for PCR-based analysis of plant, fungal, and bacterial DNA. Plant Molecular Biology Reporter. 2004. 22:71–81.8. Merk S, Meyer H, Greiser-Wilke I, Sprague LD, Neubauer H. Detection of Burkholderia cepacia DNA from artificially infected EDTA-blood and lung tissue comparing different DNA isolation methods. J Vet Med B Infect Dis Vet Public Health. 2006. 53:281–285.
Article9. Queipo-Ortuño MI, Tena F, Colmenero JD, Morata P. Comparison of seven commercial DNA extraction kits for the recovery of Brucella DNA from spiked human serum samples using real-time PCR. Eur J Clin Microbiol infect Dis. 2008. 27:109–114.10. Beuselinck K, van Ranst M, van Eldere J. Automated extraction of viral-pathogen RNA and DNA for high-throughput quantitative real-time PCR. J Clin Microbiol. 2005. 43:5541–5546.11. Leb V, Stöcher M, Valentine-Thon E, Hölzl G, Kessler H, Stekel H, et al. Fully automated, internally controlled quantification of hepatitis B Virus DNA by real-time PCR by use of the MagNA Pure LC and LightCycler instruments. J Clin Microbiol. 2004. 42:585–590.
Article12. Tang YW, Sefers SE, Li H, Kohn DJ, Procop GW. Comparative evaluation of three commercial systems for nucleic acid extraction from urine specimens. J Clin Microbiol. 2005. 43:4830–4833.
Article13. Knepp JH, Geahr MA, Forman MS, Valsamakis A. Comparison of automated and manual nucleic acid extraction methods for detection of enterovirus RNA. J Clin Microbiol. 2003. 41:3532–3536.14. Konnick EQ, Williams SM, Ashwood ER, Hillyard DR. Evaluation of the COBAS Hepatitis C Virus (HCV) TaqMan analyte-specific reagent assay and comparison to the COBAS Amplicor HCV Monitor V2.0 and Versant HCV bDNA 3.0 assays. J Clin Microbiol. 2005. 43:2133–2140.
Article15. Schrag SJ, Brooks JT, Van Beneden C, Parashar UD, Griffin PM, Anderson LJ, et al. SARS surveillance during emergency public health response, United States, March-July 2003. Emerg Infect Dis. 2004. 10:185–194.
Article16. Schuurman T, de Boer R, Patty R, Kooistra-Smid M, van Zwet A. Comparative evaluation of in-house manual, and commercial semi-automated and automated DNA extraction platforms in the sample preparation of human stool specimens for a Salmonella enterica 5'-nuclease assay. J Microbiol Methods. 2007. 71:238–245.
Article17. Kleines M, Schellenberg K, Ritter K. Efficient extraction of viral DNA and viral RNA by the Chemagic viral DNA/RNA kit allows sensitive detection of cytomegalovirus, hepatitis B virus, and hepatitis G virus by PCR. J Clin Microbiol. 2003. 41:5273–5276.18. Moss D, Harbison SA, Saul DJ. An easily automated, closed-tube forensic DNA extraction procedure using a thermostable proteinase. Int J Legal Med. 2003. 117:340–349.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Automated Nucleic Acid Extraction Systems for Detecting Cytomegalovirus and Epstein-Barr Virus Using Real-Time PCR: A Comparison Study Between the QIAsymphony RGQ and QIAcube Systems
- Nucleic Acid Extraction for the Quantification of Cytomegalovirus and Epstein-Barr Virus
- False positive cases in automated blood culture systems due to hyperleukocytosis: a case report
- Diagnostic Classification and Genomic Analyses of Cancer
- Comparison of Analytical Performance between the Sysmex UF-100 flow cytometer and the Iris iQ200 Urine Microscopy System