Lab Med Online.
2021 Oct;11(4):223-229. 10.47429/lmo.2021.11.4.223.
Diagnostic Classification and Genomic Analyses of Cancer
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Samsung Medical Center, School of Medicine, Sungkyunkwan University, Seoul, Korea
- KMID: 2526079
- DOI: http://doi.org/10.47429/lmo.2021.11.4.223
Abstract
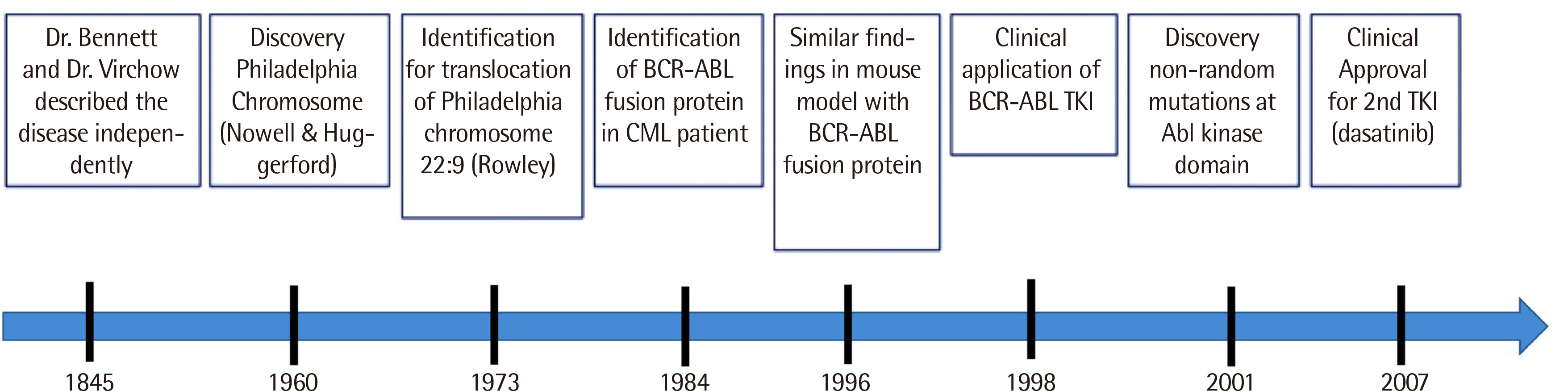
- Diagnostic classification of diseases is fundamental to disease diagnosis and treatment. Cancers have traditionally been classified based on histopathology, but cancer is defined as a genetic disease. Emerging genetic knowledge and genomic techniques applied to cancer are changing the diagnostic paradigm. Successes with targeted drug treatments such as Imatinib for chronic myeloid leukemia and Herceptin for breast cancer are accelerating the shift to the clinical genetic analysis of cancer. A recent cancer classification study using The Cancer Genome Atlas (TCGA) data yielded better performance than traditional histopathology. Eventually, genomic analysis will thoroughly reform cancer classification and diagnostic criteria.
Figure
Reference
-
1. National Research Council Committee on A Framework for Developing a New Taxonomy of Disease. 2011. Toward precision medicine: building a knowledge network for biomedical research and a new taxonomy of disease. National Academies Press;Washington, DC:2. Haendel MA, Chute CG, Robinson PN. 2018; Classification, ontology, and precision medicine. N Engl J Med. 379:1452–62. DOI: 10.1056/NEJMra1615014. PMID: 30304648. PMCID: PMC6503847.
Article3. Connolly JL, Schnitt SJ, editors. 2003. Role of the surgical pathologist in the diagnosis and management of the cancer patient. 6th ed. Holland-Frei Cancer Medicine;Hamilton, ON:4. Kinzler KW, Vogelstein B. 1996; Lessons from hereditary colorectal cancer. Cell. 87:159–70. DOI: 10.1016/S0092-8674(00)81333-1. PMID: 8861899.
Article5. Goldman JM. 2010; Chronic myeloid leukemia: a historical perspective. Semin Hematol. 47:302–11. DOI: 10.1053/j.seminhematol.2010.07.001. PMID: 20875546.
Article6. Claudiani S, Apperley JF. 2018; The argument for using imatinib in CML. Hematology Am Soc Hematol Educ Program. 2018:161–7. DOI: 10.1182/asheducation-2018.1.161. PMID: 30504305. PMCID: PMC6246007.
Article7. Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, et al. 2017; Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 376:917–27. DOI: 10.1056/NEJMoa1609324. PMID: 28273028. PMCID: PMC5901965.
Article8. O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. 2003; Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 348:994–1004. DOI: 10.1056/NEJMoa022457. PMID: 12637609.9. Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boqué C, et al. 2016; Final 5-year study results of DASISION: The dasatinib versus imatinib study in treatment-naïve chronic myeloid leukemia patients trial. J Clin Oncol. 34:2333–40. DOI: 10.1200/JCO.2015.64.8899. PMID: 27217448. PMCID: PMC5118045.
Article10. Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim DW, Issaragrisil S, et al. 2016; Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 30:1044–54. DOI: 10.1038/leu.2016.5. PMID: 26837842. PMCID: PMC4858585.
Article11. Saussele S, Hehlmann R, Fabarius A, Jeromin S, Proetel U, Rinaldetti S, et al. 2018; Defining therapy goals for major molecular remission in chronic myeloid leukemia: results of the randomized CML Study IV. Leukemia. 32:1222–8. DOI: 10.1038/s41375-018-0055-7. PMID: 29479070. PMCID: PMC5940636.
Article12. Veronesi U, Saccozzi R, Del Vecchio M, Banfi A, Clemente C, De Lena M, et al. 1981; Comparing radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast. N Engl J Med. 305:6–11. DOI: 10.1056/NEJM198107023050102. PMID: 7015141.
Article13. Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. 2002; Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 347:1227–32. DOI: 10.1056/NEJMoa020989. PMID: 12393819.
Article14. Early Breast Cancer Trialists' Collaborative Group. 1998; Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 351:1451–67. DOI: 10.1016/S0140-6736(97)11423-4. PMID: 9605801.15. Waks AG, Winer EP. 2019; Breast cancer treatment: A review. JAMA. 321:288–300. DOI: 10.1001/jama.2018.19323. PMID: 30667505.16. Pegram MD, Pauletti G, Slamon DJ. 1998; HER-2/neu as a predictive marker of response to breast cancer therapy. Breast Cancer Res Treat. 52:65–77. DOI: 10.1023/A:1006111117877. PMID: 10066073.
Article17. Loibl S, Gianni L. 2017; HER2-positive breast cancer. Lancet. 389:2415–29. DOI: 10.1016/S0140-6736(16)32417-5. PMID: 27939064.
Article18. Malhotra GK, Zhao X, Band H, Band V. 2010; Histological, molecular and functional subtypes of breast cancers. Cancer Biol Ther. 10:955–60. DOI: 10.4161/cbt.10.10.13879. PMID: 21057215. PMCID: PMC3047091.
Article19. Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. 2017; Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 377:523–33. DOI: 10.1056/NEJMoa1706450. PMID: 28578601.
Article20. Hutter C, Zenklusen JC. 2018; the cancer genome atlas: Creating lasting value beyond its data. Cell. 173:283–5. DOI: 10.1016/j.cell.2018.03.042. PMID: 29625045.
Article21. Tomczak K, Czerwińska P, Wiznerowicz M. 2015; The Cancer Genome Atlas (TCGA): an immeasurable source of knowledge. Contemp Oncol (Pozn). 19:A68–77. DOI: 10.5114/wo.2014.47136. PMID: 25691825. PMCID: PMC4322527.22. National Cancer Institute at the National Institutes of Health. https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga/publications. Last accessed on Jun 2021.23. Hoadley KA, Yau C, Wolf DM, Cherniack AD, Tamborero D, Ng S, et al. 2014; Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell. 158:929–44. DOI: 10.1016/j.cell.2014.06.049. PMID: 25109877. PMCID: PMC4152462.
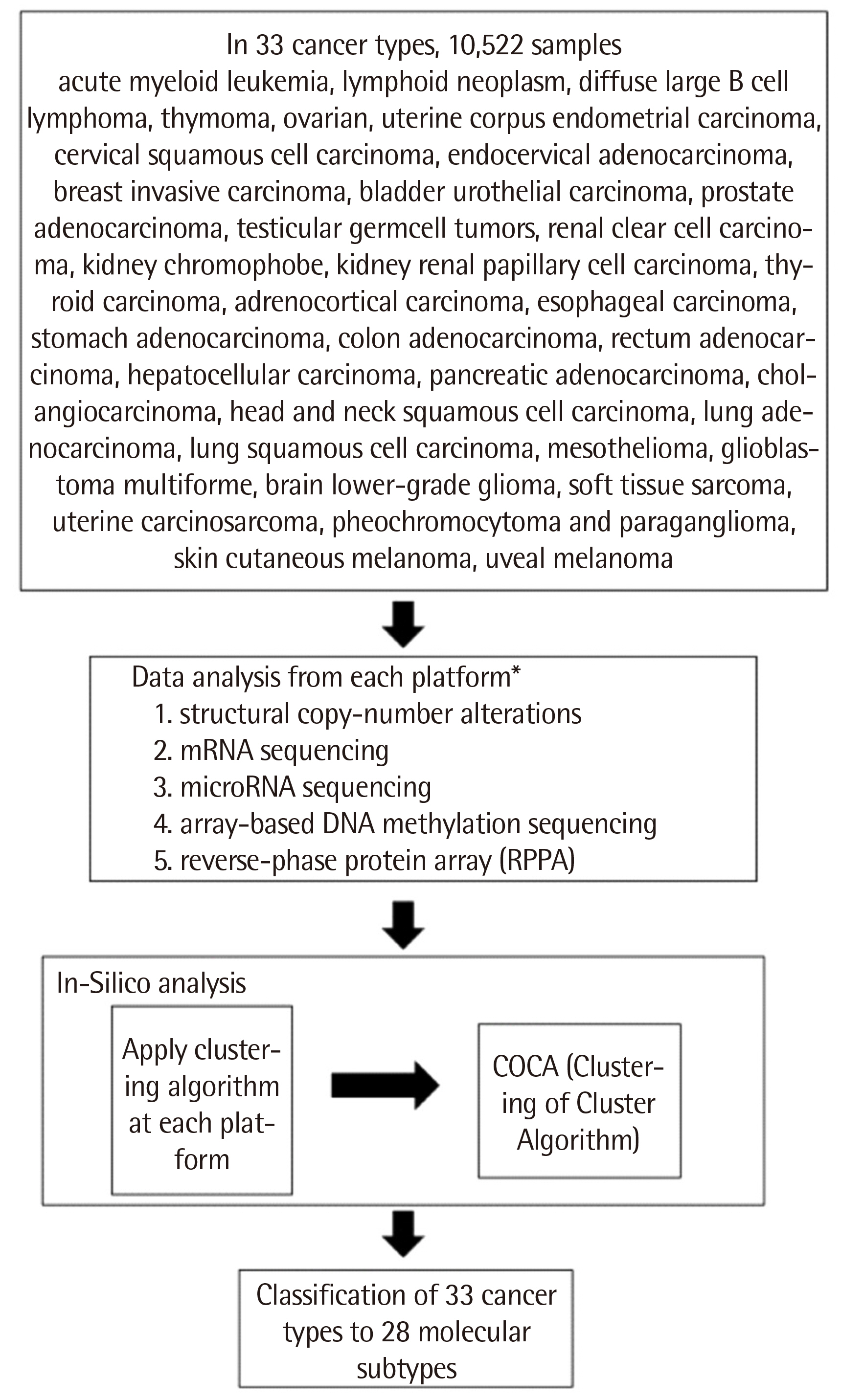
Article24. Hoadley KA, Yau C, Hinoue T, Wolf DM, Lazar AJ, Drill E, et al. 2018; Cell-of-origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer. Cell. 173:291–304.e6. DOI: 10.1016/j.cell.2018.03.022. PMID: 29625048. PMCID: PMC5957518.25. Ricketts CJ, De Cubas AA, Fan H, Smith CC, Lang M, Reznik E, et al. 2018; The cancer genome atlas comprehensive molecular characterization of renal cell carcinoma. Cell Rep. 23:313–26.e5. DOI: 10.1016/j.celrep.2018.03.075. PMID: 29617669. PMCID: PMC6075733.26. Liu Y, Sethi NS, Hinoue T, Schneider BG, Cherniack AD, Sanchez-Vega F, et al. 2018; Comparative molecular analysis of gastrointestinal adenocarcinomas. Cancer Cell. 33:721–35.e8. DOI: 10.1016/j.ccell.2018.03.010. PMID: 29622466. PMCID: PMC5966039.27. Campbell JD, Yau C, Bowlby R, Liu Y, Brennan K, Fan H, et al. 2018; Genomic, pathway network, and immunologic features distinguishing squamous carcinomas. Cell Rep. 23:194–212.e6. DOI: 10.1016/j.celrep.2018.03.063. PMID: 29617660. PMCID: PMC6002769.28. Berger AC, Korkut A, Kanchi RS, Hegde AM, Lenoir W, Liu W, et al. 2018; A comprehensive pan-cancer molecular study of gynecologic and breast cancers. Cancer Cell. 33:690–705.e9. DOI: 10.1016/j.ccell.2018.03.014. PMID: 29622464. PMCID: PMC5959730.29. Taylor AM, Shih J, Ha G, Gao GF, Zhang X, Berger AC, et al. 2018; Genomic and functional approaches to understanding cancer aneuploidy. Cancer Cell. 33:676–89.e3. DOI: 10.1016/j.ccell.2018.03.007. PMID: 29622463. PMCID: PMC6028190.30. Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, et al. 2018; The immune landscape of cancer. Immunity. 48:812–30.e14.31. Arora A, Olshen AB, Seshan VE, Shen R. 2020; Pan-cancer identification of clinically relevant genomic subtypes using outcome-weighted integrative clustering. Genome Med. 12:110. DOI: 10.1186/s13073-020-00804-8. PMID: 33272320. PMCID: PMC7716509.
Article32. Bai X, Yang X, Wu L, Zuo B, Lin J, Wang S, et al. 2019; CMTTdb: the cancer molecular targeted therapy database. Ann Transl Med. 7:667. DOI: 10.21037/atm.2019.10.23. PMID: 31930068. PMCID: PMC6944590.
Article33. Sanchez-Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC, et al. 2018; oncogenic signaling pathways in the cancer genome atlas. Cell. 173:321–37.e10. DOI: 10.1016/j.cell.2018.03.035. PMID: 29625050. PMCID: PMC6070353.34. Postow MA, Callahan MK, Wolchok JD. 2015; Immune checkpoint blockade in cancer therapy. J Clin Oncol. 33:1974–82. DOI: 10.1200/JCO.2014.59.4358. PMID: 25605845. PMCID: PMC4980573.
Article35. Huang FW, Feng FY. 2019; A tumor-agnostic NTRK (TRK) inhibitor. Cell. 177:8. DOI: 10.1016/j.cell.2019.02.049. PMID: 30901551.
Article36. Sartore-Bianchi A, Pizzutilo EG, Marrapese G, Tosi F, Cerea G, Siena S. 2020; Entrectinib for the treatment of metastatic NSCLC: safety and efficacy. Expert Rev Anticancer Ther. 20:333–41. DOI: 10.1080/14737140.2020.1747439. PMID: 32223357.
Article37. Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D, et al. 2012; Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 483:100–3. DOI: 10.1038/nature10868. PMID: 22281684.
Article38. U.S. Department of Health and Human Services Food and Drug Administrations. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-sotorasib-kras-g12c-mutated-nsclc. Updated on May 2021.39. Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, et al. 2020; KRASG12C inhibition with sotorasib in advanced solid tumors. N Engl J Med. 383:1207–17. DOI: 10.1056/NEJMoa1917239. PMID: 32955176. PMCID: PMC7571518.
Article40. Corcoran RB, Ebi H, Turke AB, Coffee EM, Nishino M, Cogdill AP, et al. 2012; EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2:227–35. DOI: 10.1158/2159-8290.CD-11-0341. PMID: 22448344. PMCID: PMC3308191.41. Photopoulos J. A hopeful revolution in cancer care. https://media.nature.com/original/magazine-assets/d41586-020-02679-6/d41586-020-02679-6.pdf. Updated on Sep 2020.