Ann Lab Med.
2017 Mar;37(2):129-136. 10.3343/alm.2017.37.2.129.
Automated Nucleic Acid Extraction Systems for Detecting Cytomegalovirus and Epstein-Barr Virus Using Real-Time PCR: A Comparison Study Between the QIAsymphony RGQ and QIAcube Systems
- Affiliations
-
- 1Department of Laboratory Medicine, Konkuk University School of Medicine, Seoul, Korea. dearmina@hanmail.net
- 2Department of Laboratory Medicine, Hallym University College of Medicine, Seoul, Korea.
- KMID: 2373630
- DOI: http://doi.org/10.3343/alm.2017.37.2.129
Abstract
- BACKGROUND
Cytomegalovirus (CMV) and Epstein-Barr virus (EBV) are increasingly important in immunocompromised patients. Nucleic acid extraction methods could affect the results of viral nucleic acid amplification tests. We compared two automated nucleic acid extraction systems for detecting CMV and EBV using real-time PCR assays.
METHODS
One hundred and fifty-three whole blood (WB) samples were tested for CMV detection, and 117 WB samples were tested for EBV detection. Viral nucleic acid was extracted in parallel by using QIAsymphony RGQ and QIAcube (Qiagen GmbH, Germany), and real-time PCR assays for CMV and EBV were performed with a Rotor-Gene Q real-time PCR cycler (Qiagen). Detection rates for CMV and EBV were compared, and agreements between the two systems were analyzed.
RESULTS
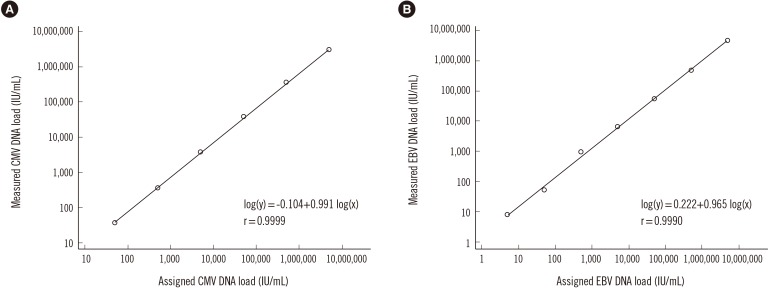
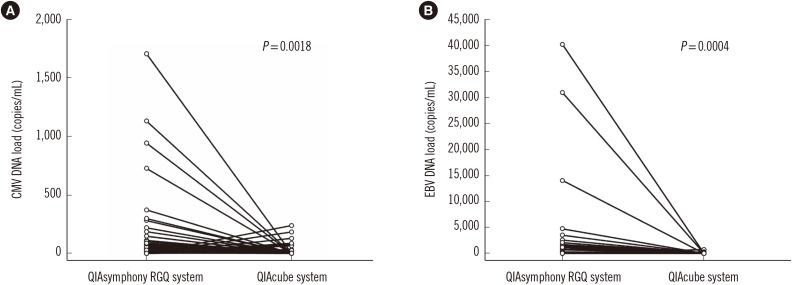
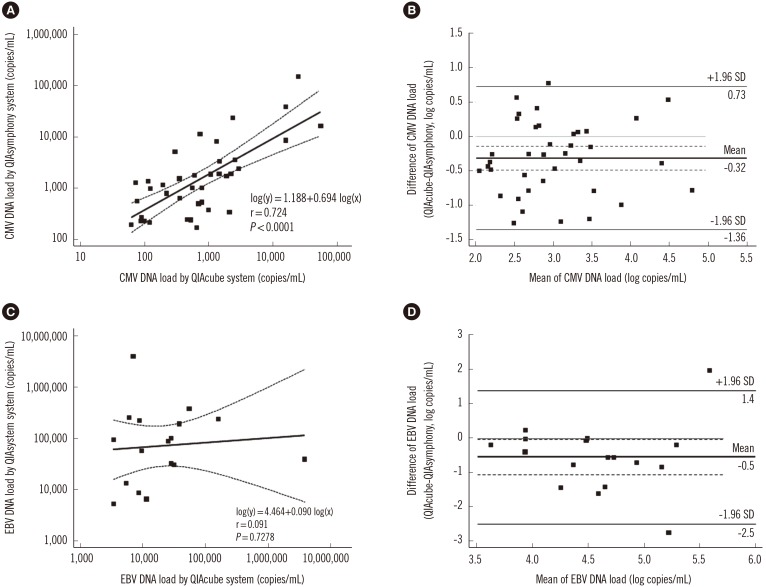
The detection rate of CMV and EBV differed significantly between the QIAsymphony RGQ and QIAcube systems (CMV, 59.5% [91/153] vs 43.8% [67/153], P=0.0005; EBV, 59.0% [69/117] vs 42.7% [50/117], P=0.0008). The two systems showed moderate agreement for CMV and EBV detection (kappa=0.43 and 0.52, respectively). QIAsymphony RGQ showed a negligible correlation with QIAcube for quantitative EBV detection. QIAcube exhibited EBV PCR inhibition in 23.9% (28/117) of samples.
CONCLUSIONS
Automated nucleic acid extraction systems have different performances and significantly affect the detection of viral pathogens. The QIAsymphony RGQ system appears to be superior to the QIAcube system for detecting CMV and EBV. A suitable sample preparation system should be considered for optimized nucleic acid amplification in clinical laboratories.
Keyword
MeSH Terms
-
Automation
Cytomegalovirus/*genetics/isolation & purification
Cytomegalovirus Infections/diagnosis/*virology
DNA, Viral/*blood/isolation & purification/metabolism
Herpesvirus 4, Human/*genetics/isolation & purification
Humans
Reagent Kits, Diagnostic
Real-Time Polymerase Chain Reaction
DNA, Viral
Reagent Kits, Diagnostic
Figure
Reference
-
1. Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010; 20:202–213. PMID: 20564615.2. Chen CY, Huang KY, Shen JH, Tsao KC, Huang YC. A large-scale seroprevalence of Epstein-Barr virus in Taiwan. PLoS One. 2015; 10:e0115836. PMID: 25615611.3. Kanakry JA, Hegde AM, Durand CM, Massie AB, Greer AE, Ambinder RF, et al. The clinical significance of EBV DNA in the plasma and peripheral blood mononuclear cells of patients with or without EBV diseases. Blood. 2016; 127:2007–2017. PMID: 26744460.4. Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Danziger-Isakov L, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013; 96:333–360. PMID: 23896556.5. Gulley ML, Tang W. Using Epstein-Barr viral load assays to diagnose, monitor, and prevent posttransplant lymphoproliferative disorder. Clin Microbiol Rev. 2010; 23:350–366. PMID: 20375356.6. Thatcher SA. DNA/RNA preparation for molecular detection. Clin Chem. 2015; 61:89–99. PMID: 25451869.7. Rahman MM, Elaissari A. Nucleic acid sample preparation for in vitro molecular diagnosis: from conventional techniques to biotechnology. Drug Discov Today. 2012; 17:1199–1207. PMID: 22819926.8. Koidl C, Bozic M, Marth E, Kessler HH. Detection of CMV DNA: is EDTA whole blood superior to EDTA plasma? J Virol Methods. 2008; 154:210–212. PMID: 18789976.9. Jones S, Webb EM, Barry CP, Choi WS, Abravaya KB, Schneider GJ, et al. Commutability of cytomegalovirus WHO international standard in different matrices. J Clin Microbiol. 2016; 54:1512–1519. PMID: 27030491.10. Hakim H, Gibson C, Pan J, Srivastava K, Gu Z, Bankowski MJ, et al. Comparison of various blood compartments and reporting units for the detection and quantification of Epstein-Barr virus in peripheral blood. J Clin Microbiol. 2007; 45:2151–2155. PMID: 17494720.11. Verheyen J, Kaiser R, Bozic M, Timmen-Wego M, Maier BK, Kessler HH. Extraction of viral nucleic acids: comparison of five automated nucleic acid extraction platforms. J Clin Virol. 2012; 54:255–259. PMID: 22503856.12. Costa C, Mantovani S, Balloco C, Sidoti F, Fop F, Cavallo R. Comparison of two nucleic acid extraction and testing systems for HCMV-DNA detection and quantitation on whole blood specimens from transplant patients. J Virol Methods. 2013; 193:579–582. PMID: 23924805.13. Miller S, Seet H, Khan Y, Wright C, Nadarajah R. Comparison of QIAGEN automated nucleic acid extraction methods for CMV quantitative PCR testing. Am J Clin Pathol. 2010; 133:558–563. PMID: 20231608.14. Pillet S, Bourlet T, Pozzetto B. Comparative evaluation of the QIAsymphony RGQ system with the easyMAG/R-gene combination for the quantitation of cytomegalovirus DNA load in whole blood. Virol J. 2012; 9:231. PMID: 23046712.15. Forman M, Wilson A, Valsamakis A. Cytomegalovirus DNA quantification using an automated platform for nucleic acid extraction and real-time PCR assay setup. J Clin Microbiol. 2011; 49:2703–2705. PMID: 21562101.16. Raggam RB, Bozic M, Salzer HJ, Hammerschmidt S, Homberg C, Ruzicka K, et al. Rapid quantitation of cytomegalovirus DNA in whole blood by a new molecular assay based on automated sample preparation and real-time PCR. Med Microbiol Immunol. 2010; 199:311–316. PMID: 20559848.17. Lee AV, Atkinson C, Manuel RJ, Clark DA. Comparative evaluation of the QIAGEN QIAsymphony® SP system and bioMérieux NucliSens easyMAG automated extraction platforms in a clinical virology laboratory. J Clin Virol. 2011; 52:339–343. PMID: 22014618.18. Buelow D, Sun Y, Tang L, Gu Z, Pounds S, Hayden R. Comparative evaluation of four real-time PCR methods for the quantitative detection of Epstein-Barr virus from whole blood specimens. J Mol Diagn. 2016; 18:527–534. PMID: 27157323.19. Laus S, Kingsley LA, Green M, Wadowsky RM. Comparison of QIAsymphony automated and QIAamp manual DNA extraction systems for measuring Epstein-Barr virus DNA load in whole blood using real-time PCR. J Mol Diagn. 2011; 13:695–700. PMID: 21889612.20. Raggam RB, Wagner J, Bozic M, Michelin BD, Hammerschmidt S, Homberg C, et al. Detection and quantitation of Epstein-Barr virus (EBV) DNA in EDTA whole blood samples using automated sample preparation and real time PCR. Clin Chem Lab Med. 2010; 48:413–418. PMID: 20001852.21. Semenova T, Lupo J, Alain S, Perrin-Confort G, Grossi L, Dimier J, et al. Multicenter evaluation of whole-blood Epstein-Barr viral load standardization using the WHO international standard. J Clin Microbiol. 2016; 54:1746–1750. PMID: 27076661.22. CLSI. Evaluation of detection capability for clinical laboratory measurement procedures; approved guideline-Second ed. CLSI document EP17-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2012.23. Mukaka MM. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012; 24:69–71. PMID: 23638278.24. Schrader C, Schielke A, Ellerbroek L, Johne R. PCR inhibitors - occurrence, properties and removal. J Appl Microbiol. 2012; 113:1014–1026. PMID: 22747964.25. Pang XL, Fox JD, Fenton JM, Miller GG, Caliendo AM, Preiksaitis JK, et al. Interlaboratory comparison of cytomegalovirus viral load assays. Am J Transplant. 2009; 9:258–268. PMID: 19178413.26. Wolff DJ, Heaney DL, Neuwald PD, Stellrecht KA, Press RD. Multi-Site PCR-based CMV viral load assessment-assays demonstrate linearity and precision, but lack numeric standardization: a report of the association for molecular pathology. J Mol Diagn. 2009; 11:87–92. PMID: 19225134.27. Gärtner B, Preiksaitis JK. EBV viral load detection in clinical virology. J Clin Virol. 2010; 48:82–90. PMID: 20395167.28. Razonable RR, Hayden RT. Clinical utility of viral load in management of cytomegalovirus infection after solid organ transplantation. Clin Microbiol Rev. 2013; 26:703–727. PMID: 24092851.29. First WHO International Standard for Epstein–Barr Virus for Nucleic Acid Amplification Techniques. Updated on Sep 2014. https://www.nibsc.org/documents/ifu/09-260.pdf.30. Hirsch HH, Lautenschlager I, Pinsky BA, Cardeñoso L, Aslam S, Cobb B, et al. An international multicenter performance analysis of cytomegalovirus load tests. Clin Infect Dis. 2013; 56:367–373. PMID: 23097587.31. Hayden RT, Yan X, Wick MT, Rodriguez AB, Xiong X, Ginocchio CC, et al. Factors contributing to variability of quantitative viral PCR results in proficiency testing samples: a multivariate analysis. J Clin Microbiol. 2012; 50:337–345. PMID: 22116152.32. Park JE, Kim JY, Yun SA, Lee MK, Huh HJ, Kim JW, Ki CS. Performance evaluation of the Real-Q cytomegalovirus (CMV) Quantification Kit using two real-time PCR systems for quantifying CMV DNA in whole blood. Ann Lab Med. 2016; 36:603–606. PMID: 27578516.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nucleic Acid Extraction for the Quantification of Cytomegalovirus and Epstein-Barr Virus
- Performance Evaluation of the ELITe InGenius System for Detecting Cytomegalovirus, EpsteinBarr Virus, and BK Virus Infections
- Performance of the Real-Q EBV Quantification Kit for Epstein-Barr Virus DNA Quantification in Whole Blood
- Evaluation of ExiPrep16 Automated System for the Extraction of Nucleic Acids from Nasopharyngeal Swabs for the Detection of Respiratory Viruses
- Performance Evaluation of the Real-Q Cytomegalovirus (CMV) Quantification Kit Using Two Real-Time PCR Systems for Quantifying CMV DNA in Whole Blood