J Korean Med Sci.
2009 Aug;24(4):684-689. 10.3346/jkms.2009.24.4.684.
Expressions of Uroplakins in the Mouse Urinary Bladder with Cyclophosphamide-Induced Cystitis
- Affiliations
-
- 1Department of Urology, Dankook University College of Medicine, Cheonan, Korea. multiorigins@yahoo.com
- KMID: 1779204
- DOI: http://doi.org/10.3346/jkms.2009.24.4.684
Abstract
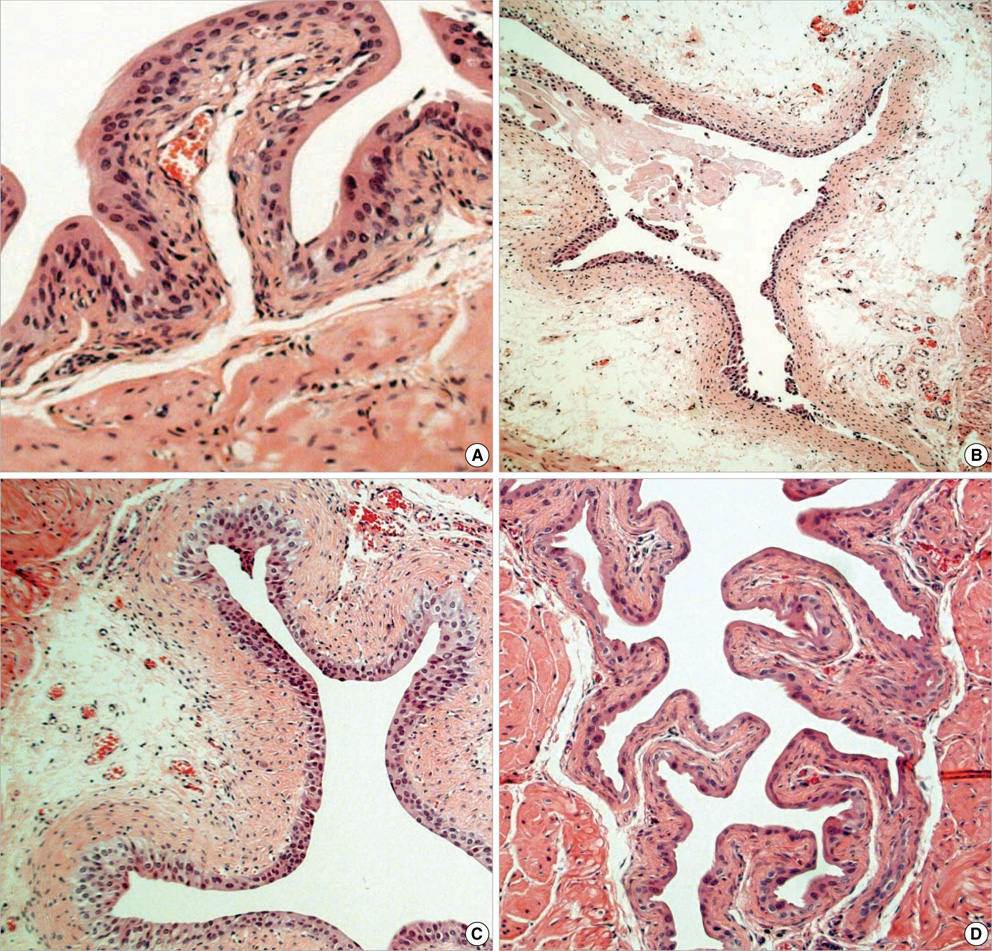
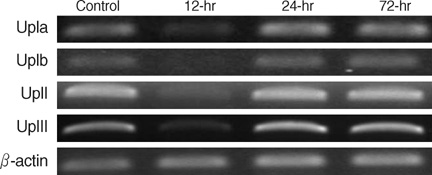
- Even though uroplakins (UPs) are believed to serve a strong protective barrier against toxic materials, cyclophosphamide (CP) causes extensive cystitis. We investigated the expression of UPs in the urothelium in CP induced mouse cystitis. A total of 27 ICR female mice received a single intraperitoneal injection of 200 mg CP/kg. Nine CP-treated mice and 6 controls were sequentially killed at 12, 24, and 72 hr post injection. Extensive cystitis and an increased vesical weight were seen. These all peaked within 12 hr post injection and they tended to decrease thereafter. The level of all the UPs mRNA, the protein expressions of UP II and III on immunoblotting study, and the expression of UP III on immunolocalization study were maximally suppressed within 12 hr; this partially recovered at 24 hr, and this completely recovered at 72 hr post CP injection. In conclusion, CP reduced the expression of UPs. The reduction of the UPs mRNA and protein was time dependent, and this peaked within 12 hr after CP injection. However, the damage was rapidly repaired within 24 hr. This study demonstrates a dynamic process, an extensive reduction and rapid recovery, for the UPs expression of the mouse urinary bladder after CP injection.
Keyword
MeSH Terms
Figure
Reference
-
1. Hornberger J, Reyes C, Lubeck D, Valente N. Economic evaluation of rituximab plus cyclophosphamide, vincristine and prednisolone for advanced follicular lymphoma. Leuk Lymphoma. 2008. 49:227–236.
Article2. Sahebi F, Copelan E, Crilley P, Bolwell B, Avalos B, Klein J, Territo M, Gajewski J. Unrelated allogeneic bone marrow transplantation using high-dose busulfan and cyclophosphamide (BU-CY) for the preparative regimen. Bone Marrow Transplant. 1996. 17:685–689.3. Letendre L, Hoagland HC, Gertz MA. Hemorrhagic cystitis complicating bone marrow transplantation. Mayo Clin Proc. 1992. 67:128–130.
Article4. Giné E, Rovira M, Real I, Burrel M, Montaña J, Carreras E, Montserrat E. Successful treatment of severe hemorrhagic cystitis after hemopoietic cell transplantation by selective embolization of the vesical arteries. Bone Marrow Transplant. 2003. 31:923–925.
Article5. Wantuch C, Piesla M, Leventhal L. Pharmacological validation of a model of cystitis pain in the mouse. Neurosci Lett. 2007. 421:250–252.
Article6. Cox PJ. Cyclophosphamide cystitis--identification of acrolein as the causative agent. Biochem Pharmacol. 1979. 28:2045–2049.
Article7. Fukushima S, Arai M, Cohen SM, Jacobs JB, Friedell GH. Scanning electron microscopy of cyclophosphamide-induced hyperplasia of the rat urinary bladder. Lab Invest. 1981. 44:89–96.8. Katz A, Epelman S, Anelli A, Gorender EF, Cruz SM, Oliveira RM, Marques LA. A prospective randomized evaluation of three schedules of mesna administration in patients receiving an ifosfamide-containing chemotherapy regimen: sustained efficiency and simplified administration. J Cancer Res Clin Oncol. 1995. 121:128–131.
Article9. Elias A, Ryan L, Sulkes A, Collins J, Aisner J, Antman KH. Response to mesna, doxorubicin, ifosfamide, and dacarbazine in 108 patients with metastatic or unresectable sarcoma and no prior chemotherapy. J Clin Oncol. 1989. 7:1208–1216.
Article10. Negrete HO, Lavelle JP, Berg J, Lewis SA, Zeidel ML. Permeability properties of the intact mammalian bladder epithelium. Am J Physiol. 1996. 271(4 Pt 2):F886–F894.
Article11. Wu XR, Manabe M, Yu J, Sun TT. Large scale purification and immunolocalization of bovine uroplakins I, II, and III. Molecular markers of urothelial differentiation. J Biol Chem. 1990. 265:19170–19179.
Article12. Wu XR, Medina JJ, Sun TT. Selective interactions of UPIa and UPIb, two members of the transmembrane 4 superfamily, with distinct single transmembrane-domained proteins in differentiated urothelial cells. J Biol Chem. 1995. 270:29752–29759.
Article13. Hu P, Deng FM, Liang FX, Hu CM, Auerbach AB, Shapiro E, Wu XR, Kachar B, Sun TT. Ablation of uroplakin III gene results in small urothelial plaques, urothelial leakage, and vesicoureteral reflux. J Cell Biol. 2000. 151:961–972.
Article14. Nazif O, Teichman JM, Gebhart GF. Neural upregulation in interstitial cystitis. Urology. 2007. 69(4):Suppl. 24–33.
Article15. Corrow KA, Vizzard MA. Phosphorylation of extracellular signal-regulated kinases in urinary bladder in rats with cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol. 2007. 293:R125–R134.
Article16. Levine LA, Richie JP. Urological complications of cyclophosphamide. J Urol. 989. 141:1063–1069.
Article17. Giglio D, Aronsson P, Eriksson L, Tobin G. In vitro characterization of parasympathetic and sympathetic responses in cyclophosphamide-induced cystitis in the rat. Basic Clin Pharmacol Toxicol. 2007. 100:96–108.
Article18. Giglio D, Delbro DS, Tobin G. Postjunctional modulation by muscarinic M2 receptors of responses to electrical field stimulation of rat detrusor muscle preparations. Auton Autacoid Pharmacol. 2005. 25:113–120.
Article19. Romih R, Koprivec D, Martincic DS, Jezernik K. Restoration of the rat urothelium after cyclophosphamide treatment. Cell Biol Int. 2001. 25:531–537.
Article20. Veranic P, Romih R, Jezernik K. What determines differentiation of urothelial umbrella cells? Eur J Cell Biol. 2004. 83:27–34.21. Kong XT, Deng FM, Hu P, Liang FX, Zhou G, Auerbach AB, Genieser N, Nelson PK, Robbins ES, Shapiro E, Kachar B, Sun TT. Roles of uroplakins in plaque formation, umbrella cell enlargement, and urinary tract diseases. J Cell Biol. 2004. 167:1195–1204.
Article22. Saban MR, Nguyen NB, Hammond TG, Saban R. Gene expression profiling of mouse bladder inflammatory responses to LPS, substance P, and antigen-stimulation. Am J Pathol. 2002. 160:2095–2110.
Article23. Ogawa K, Sun TT, Cohen SM. Analysis of differentiation-associated proteins in rat bladder carcinogenesis. Carcinogenesis. 1996. 17:961–965.24. Lavelle JP, Meyers SA, Ruiz WG, Buffington CA, Zeidel ML, Apodaca G. Urothelial pathophysiological changes in feline interstitial cystitis: a human model. Am J Physiol Renal Physiol. 2000. 278:F540–F553.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- High-definition ultrasound characterization of acute cyclophosphamide-induced cystitis in the mouse
- Pathomechanism of Interstitial Cystitis/Bladder Pain Syndrome and Mapping the Heterogeneity of Disease
- Expression of Caveolin-1 in Rat Urinary Bladder with Cyclophosphamide-Induced Cystitis
- Chronic Cyclophosphamide Induced Cystitis that is Improved by Mesna
- Three Cases of Cystitis Confused with Bladder Tumor on the Cystogram