J Korean Med Sci.
2009 Aug;24(4):605-613. 10.3346/jkms.2009.24.4.605.
Overexpression of X-linked Inhibitor of Apoptosis Protein (XIAP) is an Independent Unfavorable Prognostic Factor in Childhood de Novo Acute Myeloid Leukemia
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. hhkoo@skku.edu
- 2Division of Immunotherapy, Mogam Biotechnology Research Institute, Yongin, Korea.
- 3Genitourinary Cancer Branch, National Cancer Center, Ilsan, Korea.
- 4Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 1779191
- DOI: http://doi.org/10.3346/jkms.2009.24.4.605
Abstract
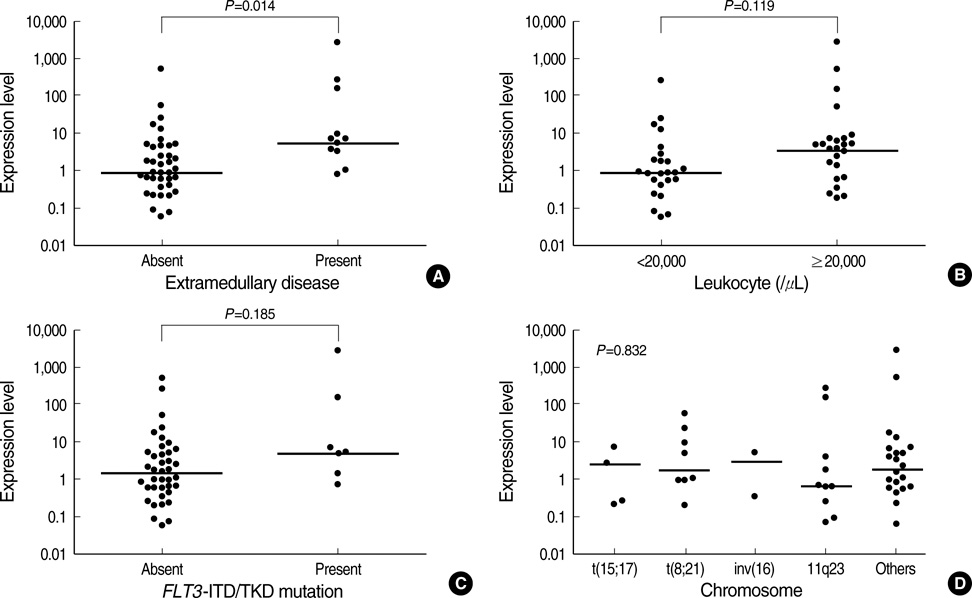
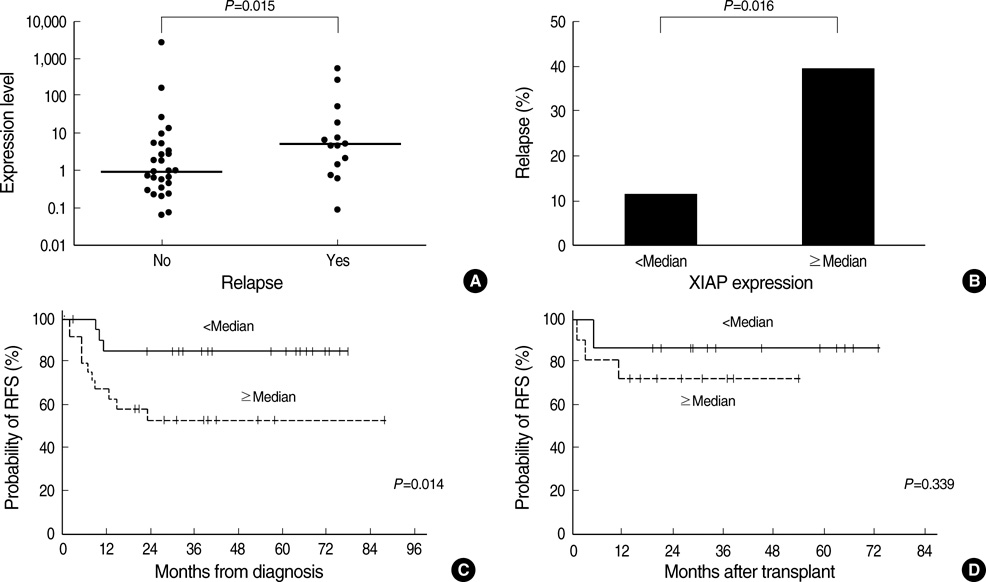
- The overexpression of X-linked inhibitor of apoptosis protein (XIAP), a member of IAP family protein, is intuitively expected to be associated with unfavorable clinical features in malignancies; however, there have been only a very limited number of studies reporting the clinical relevance of XIAP expression. This study was performed to investigate the prognostic relevance of XIAP expression in childhood acute myeloid leukemia (AML). In 53 children with de novo AML, the level of XIAP expression was determined by using quantitative reverse transcriptase-polymerase chain reaction and was analyzed with respect to the clinical characteristics at diagnosis and treatment outcomes. As a result, the XIAP expression was found to be higher in patients with extramedullary disease than in those without (P=0.014). In addition, XIAP overexpression (> or =median expression) was associated with an unfavorable day 7 response to induction chemotherapy and also associated with a worse 3-yr relapsefree survival rate (52.7+/-20.9% vs. 85.9+/-14.8%, P=0.014). Multivariate analyses revealed that XIAP overexpression was an independent unfavorable prognostic factor for relapse-free survival (hazard ratio, 6.16; 95% confidence interval, 1.48-25.74; P=0.013). Collectively, XIAP overexpression may be used as an unfavorable prognostic marker in childhood AML.
Keyword
MeSH Terms
Figure
Reference
-
1. Chang M, Raimondi SC, Ravindranath Y, Carroll AJ, Camitta B, Gresik MV, Steuber CP, Weinstein H. Prognostic factors in children and adolescents with acute myeloid leukemia (excluding children with Down syndrome and acute promyelocytic leukemia): univariate and recursive partitioning analysis of patients treated on Pediatric Oncology Group (POG) Study 8821. Leukemia. 2000. 14:1201–1207.
Article2. Grimwade D, Walker H, Harrison G, Oliver F, Chatters S, Harrison CJ, Wheatley K, Burnett AK, Goldstone AH. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001. 98:1312–1320.
Article3. Wheatley K, Burnett AK, Goldstone AH, Gray RG, Hann IM, Harrison CJ, Rees JK, Stevens RF, Walker H. A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. United Kingdom Medical Research Council's Adult and Childhood Leukaemia Working Parties. Br J Haematol. 1999. 107:69–79.4. Browman G, Preisler H, Raza A, Syracuse K, Azarnia N, Benger A, Chervenick P, D'Arrigo P, Doeblin T, Goldberg J. Use of the day 6 bone marrow to alter remission induction therapy in patients with acute myeloid leukaemia: a leukemia intergroup study. Br J Haematol. 1989. 71:493–497.
Article5. Peters WG, Willemze R, Zwaan FE, Colly LP. Day-6 bone marrow aspirate for the prediction of response to remission induction therapy for acute myelogenous leukaemia. Blut. 1988. 57:91–95.
Article6. Kern W, Haferlach T, Schoch C, Loffler H, Gassmann W, Heinecke A, Sauerland MC, Berdel W, Buchner T, Hiddemann W. Early blast clearance by remission induction therapy is a major independent prognostic factor for both achievement of complete remission and long-term outcome in acute myeloid leukemia: data from the German AML Cooperative Group (AMLCG) 1992 Trial. Blood. 2003. 101:64–70.
Article7. Golub TR. Genomic approaches to the pathogenesis of hematologic malignancy. Curr Opin Hematol. 2001. 8:252–261.
Article8. Ebert BL, Golub TR. Genomic approaches to hematologic malignancies. Blood. 2004. 104:923–932.
Article9. Herr I, Debatin KM. Cellular stress response and apoptosis in cancer therapy. Blood. 2001. 98:2603–2614.
Article10. Yang YL, Li XM. The IAP family: endogenous caspase inhibitors with multiple biological activities. Cell Res. 2000. 10:169–177.
Article11. Nachmias B, Ashhab Y, Ben-Yehuda D. The inhibitor of apoptosis protein family (IAPs): an emerging therapeutic target in cancer. Semin Cancer Biol. 2004. 14:231–243.
Article12. Choi J, Hwang YK, Sung KW, Lee SH, Yoo KH, Jung HL, Koo HH, Kim HJ, Kang HJ, Shin HY, Ahn HS. Expression of Livin, an antiapoptotic protein, is an independent favorable prognostic factor in childhood acute lymphoblastic leukemia. Blood. 2007. 109:471–477.
Article13. Choi J, Hwang YK, Sung KW, Kim DH, Yoo KH, Jung HL, Koo HH. Aven overexpression: association with poor prognosis in childhood acute lymphoblastic leuk. Leuk Res. 2006. 30:1019–1025.14. Tamm I, Kornblau SM, Segall H, Krajewski S, Welsh K, Kitada S, Scudiero DA, Tudor G, Qui YH, Monks A, Andreeff M, Reed JC. Expression and prognostic significance of IAP-family genes in human cancers and myeloid leukemias. Clin Cancer Res. 2000. 6:1796–1803.15. Tamm I, Richter S, Oltersdorf D, Creutzig U, Harbott J, Scholz F, Karawajew L, Ludwig WD, Wuchter C. High expression levels of x-linked inhibitor of apoptosis protein and survivin correlate with poor overall survival in childhood de novo acute myeloid leukemia. Clin Cancer Res. 2004. 10:3737–3744.
Article16. Wrzesien-Kus A, Smolewski P, Sobczak-Pluta A, Wierzbowska A, Robak T. The inhibitor of apoptosis protein family and its antagonists in acute leukemias. Apoptosis. 2004. 9:705–715.
Article17. Nakagawa Y, Yamaguchi S, Hasegawa M, Nemoto T, Inoue M, Suzuki K, Hirokawa K, Kitagawa M. Differential expression of survivin in bone marrow cells from patients with acute lymphocytic leukemia and chronic lymphocytic leukemia. Leuk Res. 2004. 28:487–494.
Article18. Yamamoto K, Abe S, Nakagawa Y, Suzuki K, Hasegawa M, Inoue M, Kurata M, Hirokawa K, Kitagawa M. Expression of IAP family proteins in myelodysplastic syndromes transforming to overt leukemia. Leuk Res. 2004. 28:1203–1211.
Article19. Wuchter C, Richter S, Oltersdorf D, Karawajew L, Ludwig WD, Tamm I. Differences in the expression pattern of apoptosis-related molecules between childhood and adult de novo acute myeloid leukemia. Haematologica. 2004. 89:363–364.20. Campos L, Sabido O, Sebban C, Charrin C, Bertheas MF, Fiere D, Guyotat D. Expression of BCL-2 proto-oncogene in adult acute lymphoblastic leukemia. Leukemia. 1996. 10:434–438.21. Coustan-Smith E, Kitanaka A, Pui CH, McNinch L, Evans WE, Raimondi SC, Behm FG, Arico M, Campana D. Clinical relevance of BCL-2 overexpression in childhood acute lymphoblastic leukemia. Blood. 1996. 87:1140–1146.
Article22. Hogarth LA, Hall AG. Increased BAX expression is associated with an increased risk of relapse in childhood acute lymphocytic leukemia. Blood. 1999. 93:2671–2678.
Article23. Prokop A, Wieder T, Sturm I, Essmann F, Seeger K, Wuchter C, Ludwig WD, Henze G, Dorken B, Daniel PT. Relapse in childhood acute lymphoblastic leukemia is associated with a decrease of the Bax/Bcl-2 ratio and loss of spontaneous caspase-3 processing in vivo. Leukemia. 2000. 14:1606–1613.
Article24. Rajcan-Separovic E, Liston P, Lefebvre C, Korneluk RG. Assignment of human inhibitor of apoptosis protein (IAP) genes xiap, hiap-1, hiap-2 to chromosomes Xq25 and 11q22-q23 by fluorescence in situ hybridization. Genomics. 1996. 37:404–406.25. Kaspers GJ, Veerman AJ, Pieters R, Van Zantwijk CH, Smets LA, Van Wering ER, Van Der Does-Van Den Berg A. In vitro cellular drug resistance and prognosis in newly diagnosed childhood acute lymphoblastic leukemia. Blood. 1997. 90:2723–2729.
Article26. Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, Sultan C. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann Intern Med. 1985. 103:620–625.27. Gaynon PS, Desai AA, Bostrom BC, Hutchinson RJ, Lange BJ, Nachman JB, Reaman GH, Sather HN, Steinherz PG, Trigg ME, Tubergen DG, Uckun FM. Early response to therapy and outcome in childhood acute lymphoblastic leukemia: a review. Cancer. 1997. 80:1717–1726.28. Nachman J, Sather HN, Gaynon PS, Lukens JN, Wolff L, Trigg ME. Augmented Berlin-Frankfurt-Munster therapy abrogates the adverse prognostic significance of slow early response to induction chemotherapy for children and adolescents with acute lymphoblastic leukemia and unfavorable presenting features: a report from the Children's Cancer Group. J Clin Oncol. 1997. 15:2222–2230.
Article29. Gajjar A, Ribeiro R, Hancock ML, Rivera GK, Mahmoud H, Sandlund JT, Crist WM, Pui CH. Persistence of circulating blasts after 1 week of multiagent chemotherapy confers a poor prognosis in childhood acute lymphoblastic leukemia. Blood. 1995. 86:1292–1295.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Apoptosis, Expression of p53 and p21 in Patients with Myelodysplastic Syndrome and Acute Myelogenous Leukemia
- Multi-drug resistance (MDR1) gene expression in de novo acute leukemia cells: correlations with CD surface markers and treatment outcome
- Inhibitors of Apoptosis Proteins Expression and Their Prognostic Significance in Colorectal Carcinoma
- Relationship between Expression of XIAP Protein in Operable Non- small Cell Lung Carcinomas and Apoptosis Index and Postoperative Prognosis
- Treatments for children and adolescents with AML