J Korean Med Sci.
2009 Apr;24(2):289-295. 10.3346/jkms.2009.24.2.289.
Effect of Iron-Chelator Deferiprone on the In Vitro Growth of Staphylococci
- Affiliations
-
- 1Research Center for Resistant Cells, Chosun University Medical School, Gwangju, Korea.
- 2Department of Microbiology, Chosun University Medical School, Gwangju, Korea. shsin@chosun.ac.kr
- KMID: 1779132
- DOI: http://doi.org/10.3346/jkms.2009.24.2.289
Abstract
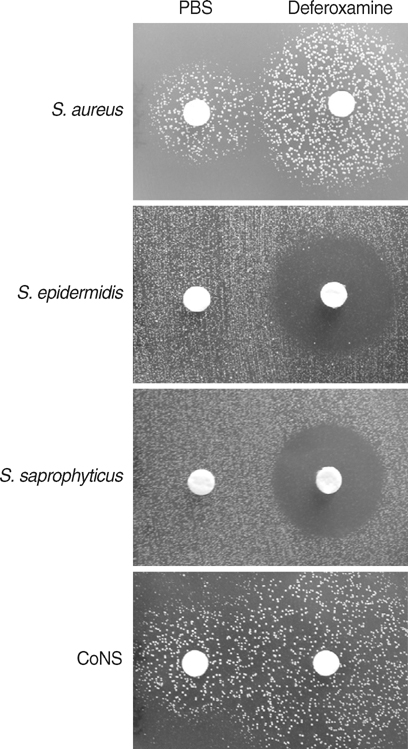
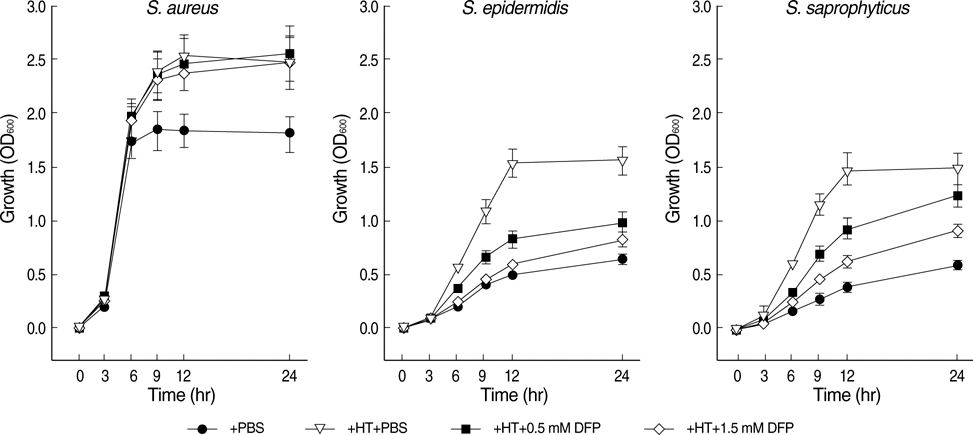
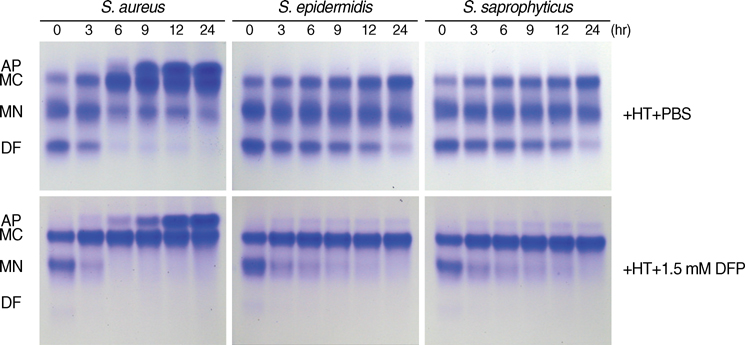
- The standard iron-chelator deferoxamine is known to prevent the growth of coagulase-negative staphylococci (CoNS) which are major pathogens in iron-overloaded patients. However, we found that deferoxamine rather promotes the growth of coagulase-positive Staphylococcus aureus. Accordingly, we tested whether deferiprone, a new clinically-available iron-chelator, can prevent the growth of S. aureus strains as well as CoNS. Deferiprone did not at least promote the growth of all S. aureus strains (n=26) and CoNS (n=27) at relatively low doses; moreover, it could significantly inhibit the growth of all staphylococci on non-transferrin-bound-iron and the growth of all CoNS on transferrin-bound iron at relatively high doses. At the same doses, it did not at least promote the growth of all S. aureus strains on transferrin-bound-iron. These findings indicate that deferiprone can be useful to prevent staphylococcal infections, as well as to improve iron overload, in iron-overloaded patients.
Keyword
MeSH Terms
Figure
Reference
-
1. Ratledge C, Dover LG. Iron metabolism in pathogenic bacteria. Ann Rev Microbiol. 2000. 54:881–941.
Article2. Brown JS, Holden DW. Iron acquisition by Gram-positive bacterial pathogens. Microbes Infect. 2002. 4:1149–1156.
Article3. Kontoghiorghes GJ, Weinberg ED. Iron: mammalian defense mechanisms, mechanisms of disease, and chelation therapy approaches. Blood Rev. 1995. 9:33–45.4. von Bonsdorff L, Tolo H, Lindeberg E, Nyman T, Harju A, Parkkinen J. Development of a pharmaceutical apotransferrin product for iron binding therapy. Biologicals. 2001. 29:27–37.
Article5. Brown JS, Ogunniyi AD, Woodrow MC, Holden DW, Paton JC. Immunization with components of two iron-uptake ABC transporters protects mice against systemic infection with Streptococcus pneumoniae. Infect Immun. 2001. 69:6702–6706.6. Etz H, Minh DB, Henics T, Dryla A, Winkler B, Triska C, Boyd AP, Sollner J, Schmidt W, von Ahsen U, Buschle M, Gill SR, Kolonay J, Khalak H, Fraser CM, von Gabain A, Nagy E, Meinke A. Identification of in vivo expressed vaccine candidate antigens from Staphylococcus aureus. Proc Natl Acad Sci USA. 2002. 99:6573–6578.
Article7. Marx JJ. Iron and infection: competition between host and microbes for a precious element. Best Pract Res Clin Haematol. 2002. 15:411–426.
Article8. Bradley SJ, Gosriwitana I, Srichairatanakool S, Hider RC, Porter JB. Non-transferrin-bound iron induced by myeloablative chemotherapy. Br J Haematol. 1997. 99:337–343.
Article9. Sahlstedt L, Ebeling F, von Bonsdorff L, Parkkinen J, Ruutu T. Non-transferrin-bound iron during allogeneic stem cell transplantation. Br J Haematol. 2001. 113:836–838.
Article10. Matinaho S, von Bonsdorff L, Rouhiainen A, Lönnroth M, Parkkinen J. Dependence of Staphylococcus epidermidis on non-transferrin-bound iron for growth. FEMS Microbiol Lett. 2003. 196:177–182.11. von Bonsdorff L, Sahlstedt L, Ebeling F, Ruutu T, Parkkinen J. Apotransferrin administration prevents growth of Staphylococcus epidermidis in serum of stem cell transplant patients by binding of free iron. FEMS Immunol Med Microbiol. 2003. 37:45–51.12. Aguila A, Herrera AG, Morrison D, Cosgrove B, Perojo A, Montesinos I, Perez J, Sierra G, Gemmell CG, Brock JH. Bacteriostatic activity of human lactoferrin against Staphylococcus aureus is a function of iron-binding properties and is not influenced by antibiotic resistance. FEMS Immunol Med Microbiol. 2001. 31:145–152.13. Olivieri NF, Brittenham GM. Iron-chelating therapy and the treatment of thalassemia. Blood. 1997. 89:739–761.
Article14. van Asbeck BS, Marcelis JH, Struyvenberg A, van Kats JH, Verhoef J. Inhibition of bacterial multiplication by the iron chelator deferoxamine; potentiating effect of ascorbic acid. Eur J Clin Microbiol. 1983. 2:426–431.
Article15. van Asbeck BS, Marcelis JH, van Kats JH, Jaarsma EY, Verhoef J. Synergy between the iron chelator deferoxamine and the antimicrobial agents gentamicin, chloramphenicol, cefalothin, cefotiam and cefsulodin. Eur J Clin Microbiol. 1983. 2:432–438.
Article16. Hartzen SH, Frimodt-Moller N, Thomsen VF. The antibacterial activity of a siderophore. 3. The activity of deferoxamine in vitro and its influence on the effect of antibiotics against Escherichia coli, Proteus mirabilis and coagulase-negative staphylococci. APMIS. 1994. 102:219–226.17. Hartzen SH, Frimodt-Moller N, Thomsen VF. The antibacterial activity of a siderophore. 2. The influence of deferoxamine alone and combined with ascorbic acid on the activity of antibiotics against Staphylococcus aureus. APMIS. 1991. 99:879–886.18. Hartzen SH, Frimodt-Moller N, Frolund Thomsen V. The antibacterial activity of a siderophore. 1. In vitro activity of deferoxamine alone and in combination with ascorbic acid on Staphylococcus aureus. APMIS. 1989. 97:419–424.19. Baumler AJ, Hantke K. Ferrioxamine uptake in Yersinia enterocolitica; characterization of the receptor protein FoxA. Mol Microbiol. 1992. 6:1309–1321.
Article20. Lesic B, Foulon J, Carniel E. Comparison of the effects of deferiprone versus deferoxamine on growth and virulence of Yersinia enterocolitica. Antimicrob Agents Chemother. 2002. 46:1741–1745.
Article21. Tanabe T, Takata N, Naka A, Moon YH, Nakao H, Inoue Y, Narimatsu S, Yamamoto S. Identification of an AraC-like regulator required for induction of the 78-kDa ferrioxamine B receptor in Vibrio vulnificus. FEMS Microbiol Lett. 2005. 249:309–314.22. Aso H, Miyoshi S, Nakao H, Okamoto K, Yamamoto S. Induction of an outer membrane protein of 78 kDa in Vibrio vulnificus in the presence of desferrioxamine B under iron-limiting conditions. FEMS Microbiol Lett. 2002. 212:65–70.23. Sebulsky MT, Hohnstein D, Hunter MD, Heinrichs DE. Identification and characterization of a membrane permease involved in iron-hydroxamate transport in Staphylococcus aureus. J Bacteriol. 2000. 182:4394–4400.24. Hoffbrand AV, Al-Refaie F, Davis B, Siritanakatkul N, Jackson BF, Cochrane J, Prescott E, Wonke B. Long-term trial of deferiprone in 51 transfusion-dependent iron overloaded patients. Blood. 1998. 91:295–300.
Article25. Barman Balfour JA, Foster RH. Deferiprone: a review of its clinical potential in iron overload in beta-thalassemia major and other transfusion-dependent diseases. Drugs. 1999. 58:553–558.26. Kim CM, Park RY, Choi MH, Sun HY, Shin SH. Ferrophilic characteristics of Vibrio vulnificus and potential usefulness of iron chelating therapy. J Infect Dis. 2007. 195:90–98.27. Lindsay JA, Riley TV, Mee BJ. Staphylococcus aureus but not Staphylococcus epidermidis can acquire iron from transferrin. Microbiology. 1995. 141:197–203.
Article28. Makey DG, Seal US. The detection of four molecular forms of human transferrin during the iron binding process. Biochem Biophys Acta. 1976. 453:250–256.
Article29. Sebulsky MT, Heinrichs DE. Identification and characterization of fhuD1 and fhuD2, two genes involved in iron-hydroxamate uptake in Staphylococcus aureus. J Bacteriol. 2001. 183:4994–5000.
Article30. Cockayne A, Hill PJ, Powell NB, Bishop K, Sims VK, Williams P. Molecular cloning of a 32-kilodalton lipoprotein component of a novel iron-regulated Staphylococcus epidermidis ABC transporter. Infect Immun. 1998. 66:3767–3774.
Article31. Park RY, Sun HY, Choi MH, Bai YH, Shin SH. Staphylococcus aureus siderophore-mediated iron-acquisition system plays a dominant and essential role in the utilization of transferrin-bound iron. J Microbiol. 2005. 43:183–190.32. Park RY, Sun HY, Choi MH, Bae YH, Shin SH. Growth of Staphylococcus aureus with defective siderophore production in human peritoneal dialysate solution. J Microbiol. 2005. 43:54–61.33. Park RY, Sun HY, Choi MH, Bai YH, Shin SH. Utilization of transferrin-bound iron by medically important staphylococcal species. J Bacteriol Virol. 2005. 35:103–112.34. Chung JH, Park MH, Kim JH, Lim Y, Shin SH. Growth and siderophore production of Staphylococci in human peritoneal dialysate. J Korean Med Sci. 2003. 18:158–162.
Article35. Trivier D, Courcol RJ. Iron-depletion and virulence in Staphylococcus aureus. FEMS Microbiol Lett. 1996. 141:117–127.36. Kontoghiorghes GJ. Iron mobilization from transferrin and non-transferrin-bound-iron by deferiprone: implications in the treatment of thalassemia, anemia of chronic disease, cancer and other conditions. Hemoglobin. 2006. 30:183–200.
Article37. al-Refaie FN, De Silva CE, Wonke B, Hoffbrand AV. Changes in transferrin saturation after treatment with the oral iron chelator deferiprone in patients with iron overload. J Clin Pathol. 1995. 48:110–114.
Article38. Kontoghiorghes GJ, Pattichis K, Neocleous K, Kolnagou A. The design and development of deferiprone (L1) and other iron chelators for clinical use: targeting methods and application prospects. Curr Med Chem. 2004. 11:2161–2183.
Article39. Ashrafian H. Hepcidin: the missing link between hemochromatosis and infections. Infect Immun. 2003. 71:6693–6700.40. Kontoghiorghes GJ, Aldouri MA, Hoffbrand AV, Barr J, Wonke B, Kourouclaris T, Sheppard L. Effective chelation of iron in β-thalassemia with the oral chelator 1,2-dimethyl-3-hydroxypyrid-4-one. Br Med J (Clin Res Ed). 1987. 295:1509–1512.41. Cappellini MD. Iron-chelating therapy with the new oral agent ICL670 (Exjade). Best Pract Res Clin Haematol. 2005. 18:289–298.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment of Pantothenate-Kinase Neurodegeneration With Baclofen, Botulinum Toxin, and Deferiprone: A Case Report
- Deferiprone Related Arthropathy in 11-Year-Old Boy with Hereditary Spherocytosis
- Utilization of Transferrin-Bound Iron by Medically Important Staphylococcal Species
- Efficacy and safety of deferiprone (Ferriprox), an oral iron-chelating agent, in pediatric patients
- A Study of Effect of Diabetic Sera on in Vitro Growth of Candida albicans