J Korean Med Sci.
2004 Dec;19(6):834-841. 10.3346/jkms.2004.19.6.834.
In vivo Recombinant Adenovirus-mediated p53 Gene Therapy in a Syngeneic Rat Model for Colorectal Cancer
- Affiliations
-
- 1Department of Surgery, Gachon Medical School, Gil Medical Center, Incheon, Korea.
- 2Department of Molecular Biology, The Cleveland Clinic Foundation, USA.
- 3Department of Clinical Pathology, The Cleveland Clinic Foundation, USA.
- 4Department of Colorectal Surgery, The Cleveland Clinic Foundation, USA.
- 5Department of Hematology & Medical Oncology, The Cleveland Clinic Foundation, USA.
- 6Department of Surgery, New York Presbyterian Hospital Weill Cornell Medical Center, USA. jwm2001@med.cornell.edu
- 7Department of Pathology, Chungnam National University, Daejon, Korea.
- 8Department of Surgery, Hanyang University, Seoul, Korea.
- KMID: 1778565
- DOI: http://doi.org/10.3346/jkms.2004.19.6.834
Abstract
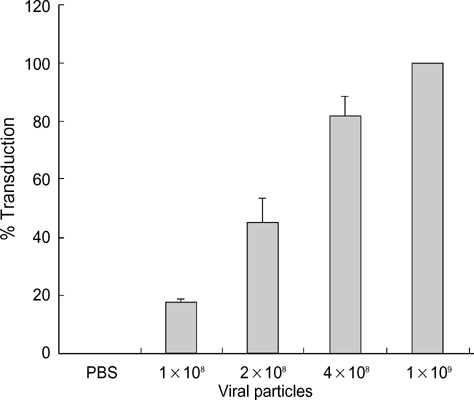
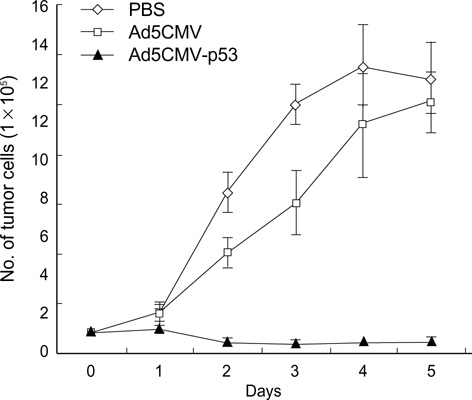
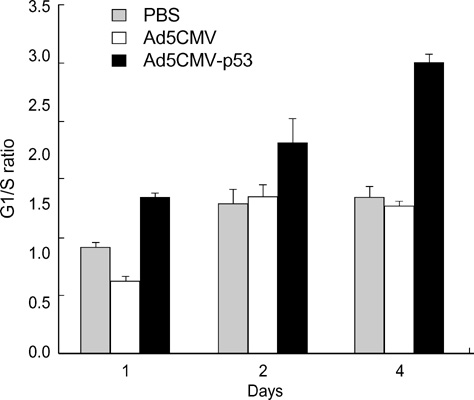
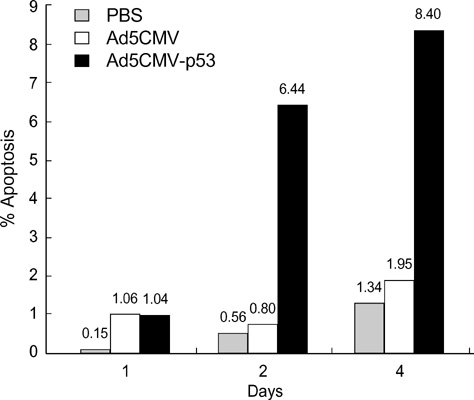
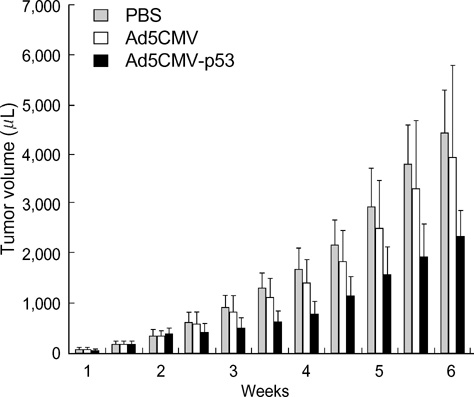
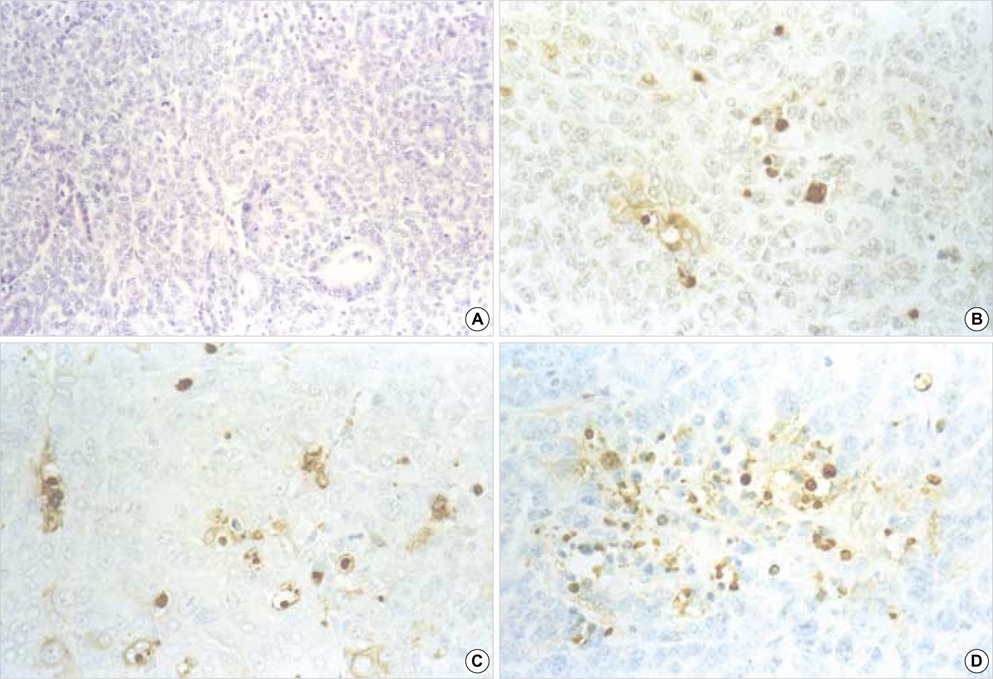
- The p53 gene has a significant role in controlling genomic stability of cancer. The purpose of this study was to evaluate the tumor response of allograft colorectal tumor treated with Ad5CMV-p53 in a syngeneic rat model. Two weeks after the inoculation of WB-2054-M5 tumor cells in the flank of rats, rats were randomly assigned by tumor size to one of three groups (n=18 in each): phosphate buffered saline (PBS), Ad5CMV, and Ad5CMV-p53. Recombinant adenovirus or PBS was administered through intratumoral injection at three divided doses every other day for 4 weeks. Apoptosis of the tumors was evaluated using TUNEL assay. After 2 and 4 weeks of treatment, the volume (cm3) of tumors in PBS, Ad5CMV, and Ad5CMV-p53 was as follows: 2 week: 1.66 +/-0.43, 1.40 +/-0.47, 0.75 +/-0.26 (p<0.001), 4 week: 4.41 +/-0.88, 3.93 +/-1.86, 2.33 +/-0.51 (p<0.001). Tumor growth showed no statistically significant difference between the PBS and Ad5CMV groups (6-week vol. p=0.32). The TUNEL assay results revealed more apparent apoptotic cells in Ad5CMV-p53-treated tumors than in other groups. Growth of allograft colorectal cancer in the syngeneic rat model was significantly suppressed by intratumoral Ad5CMV-p53 gene therapy. These results demonstrate that gene replacement therapy with p53 may provide a novel modality of treatment in conjunction with other present treatments for metastatic colorectal cancer.
MeSH Terms
-
Adenocarcinoma/genetics/pathology/therapy
Adenoviridae/*genetics
Animals
Cell Line, Tumor
Cell Proliferation
Cell Survival/genetics
Colorectal Neoplasms/*genetics/pathology/*therapy
Disease Models, Animal
Female
Gene Therapy/*methods
Gene Transfer Techniques
Men
Protein p53/*genetics/*therapeutic use
Rats
Rats, Inbred WF
Recombinant Proteins/therapeutic use
Research Support, Non-U.S. Gov't
Transplantation, Isogeneic
Treatment Outcome
Figure
Reference
-
1. Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer Statistics, 2004. CA Cancer J Clin. 2004. 54:8–29.
Article2. Finlay IG, McArdle CS. Occult hepatic metastases in colorectal carcinoma. Br J Surg. 1986. 73:732–735.
Article3. Bozzetti F, Cozzaglio L, Boracchi P, Marubini E, Doci R, Bignami P, Gennari L. Comparing surgical resection of limited hepatic metastases from colorectal cancer to non-operative treatment. Eur J Surg Oncol. 1993. 19:162–167.4. Kock H, Harris MP, Anderson SC, Machemer T, Hancock W, Sutjipto S, Wills KN, Gregory RJ, Shepard HM, Westphal M, Maneval DC. Adenovirus-mediated p53 gene transfer suppresses growth of human glioblastoma cells in vitro and in vivo. Int J Cancer. 1996. 67:808–815.5. Lane DP, Crawford LV. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979. 278:261–263.6. Lane DP, Benchimol S. p53: oncogene or anti-oncogene? Gene Dev. 1990. 4:1–8.
Article7. Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991. 253:49–53.
Article8. Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991. 351:453–456.
Article9. Bookstein R, Demers W, Gregory R, Maneval D, Park J, Wills K. p53 gene therapy in vivo of hepatocellular and liver metastatic colorectal cancer. Semin Oncol. 1996. 23:66–77.10. Baker SJ, Markowitz S, Fearon ER, Willson JK, Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990. 249:912–915.
Article11. Agarwal ML, Taylor WR, Chernov MV, Chernova OB, Stark GR. The p53 network. J Biol Chem. 1998. 273:1–4.
Article12. Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993. 74:957–967.
Article13. Spitz FR, Nguyen D, Skibber JM, Cusack J, Roth JA, Cristiano RJ. In vivo adenovirus-mediated p53 tumor suppressor gene therapy for colorectal cancer. Anticancer Res. 1996. 16:3415–3422.14. Agarwal ML, Agarwal A, Taylor WR, Stark GR. p53 controls both the G2/M and the G1 cell cycle checkpoints and mediates reversible growth arrest in human fibroblasts. Proc Natl Acad Sci USA. 1995. 92:8493–8497.
Article15. Agarwal ML, Agarwal A, Taylor WR, Wang ZQ, Wagner EF, Stark GR. Defective induction but normal activation and function of p53 in mouse cells lacking poly-ADP-ribose polymerase. Oncogene. 1997. 15:1035–1041.
Article16. Ravikumar TS, D'Emilia J, Cocchiaro C, Wolf B, King V, Steele G Jr. Experimental liver metastasis. Implications of clonal proclivity and organ specificity. Arch Surg. 1989. 124:49–54.17. Fidler IJ, Kripke ML. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977. 197:893–895.
Article18. Fidler IJ. Selection of successive tumour lines for metastasis. Nat New Biol. 1973. 242:148–149.
Article19. Liu TJ, Zhang WW, Taylor DL, Roth JA, Goepfert H, Clayman GL. Growth suppression of human head and neck cancer cells by the introduction of a wild-type p53 gene via a recombinant adenovirus. Cancer Res. 1994. 54:3662–3667.20. Boviatsis EJ, Chase M, Wei MX, Tamiya T, Hurford RK Jr, Kowall NW, Tepper RI, Breakefield XO, Chiocca EA. Gene transfer into experimental brain tumors mediated by adenovirus, herpes simplex virus, and retrovirus vectors. Hum Gene Ther. 1994. 5:183–191.
Article21. Landegren U. Measurement of cell numbers by means of the endogenous enzyme hexosaminidase. Applications to detection of lymphokines and cell surface antigens. J Immunol Methods. 1984. 67:379–388.
Article22. Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992. 119:493–501.
Article23. Bouvet M, Ellis LM, Nishizaki M, Fujiwara T, Liu W, Bucana CD, Fang B, Lee JJ, Roth JA. Adenovirus-mediated wild-type p53 gene transfer down-regulates vascular endothelial growth factor expression and inhibits angiogenesis in human colon cancer. Cancer Res. 1998. 58:2288–2292.24. Drazan KE, Csete ME, Da Shen X, Bullington D, Cottle G, Busuttil RW, Shaked A. Hepatic function is preserved following liver-directed, adenovirus-mediated gene transfer. J Surg Res. 1995. 59:299–304.
Article25. Drazan KE, Shen XD, Csete ME, Zhang WW, Roth JA, Busuttil RW, Shaked A. In vivo adenoviral-mediated human p53 tumor suppressor gene transfer and expression in rat liver after resection. Surgery. 1994. 116:197–203. discussion 203-4.26. Zhang WW, Fang X, Mazur W, French BA, Georges RN, Roth JA. High-efficiency gene transfer and high-level expression of wild-type p53 in human lung cancer cells mediated by recombinant adenovirus. Cancer Gene Ther. 1994. 1:5–13.27. Clayman GL, el-Naggar AK, Roth JA, Zhang WW, Goepfert H, Taylor DL, Liu TJ. In vivo molecular therapy with p53 adenovirus for microscopic residual head and neck squamous carcinoma. Cancer Res. 1995. 55:1–6.28. Kim J, Hwang ES, Kim JS, You EH, Lee SH, Lee JH. Intraperitoneal gene therapy with adenoviral-mediated p53 tumor suppressor gene for ovarian cancer model in nude mouse. Cancer Gene Ther. 1999. 6:172–178.
Article29. Harris MP, Sutjipto S, Wills KN, Hancock W, Cornell D, Johnson DE, Gregory RJ, Shepard HM, Maneval DC. Adenovirus-mediated p53 gene transfer inhibits growth of human tumor cells expressing mutant p53 protein. Cancer Gene Ther. 1996. 3:121–130.30. Liu TJ, el-Naggar AK, McDonnell TJ, Steck KD, Wang M, Taylor DL, Clayman GL. Apoptosis induction mediated by wild-type p53 adenoviral gene transfer in squamous cell carcinoma of the head and neck. Cancer Res. 1995. 55:3117–3122.31. Colecchia M, Frigo B, Del Boca C, Guardamagna A, Zucchi A, Colloi D, Leopardi O. Detection of apoptosis by the TUNEL technique in clinically localized prostatic cancer before and after combined endocrine therapy. J Clin Pathol. 1997. 50:384–388.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Synergistic Cell Killing Effects by the Transduction of the w-p53 Gene and 5-FU Administration in Colon Cancer Cell Lines
- Stromelysin Gene Expression in Rat Eye Mediated by the Adenovirus Vector
- Gene Therapy of Brain Tumors:Effects of Adenovirus-mediated Wild Type p53 Gene Transfer in Human Glioma Cells
- Construction of Recombinant p53 Adenovirus ( Ad5CMVp53 ) for Cervical Cancer Gene Therapy
- Effect of Adenovirus-p53 to Non-Small Cell Lung Cancer Cell Lines