J Korean Med Sci.
2004 Dec;19(6):826-833. 10.3346/jkms.2004.19.6.826.
E-cadherin and Cytokeratin Subtype Profiling in Effusion Cytology
- Affiliations
-
- 1Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Division of Endocrine Surgery, Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. sjnam@smc.samsung.co.kr
- KMID: 1778564
- DOI: http://doi.org/10.3346/jkms.2004.19.6.826
Abstract
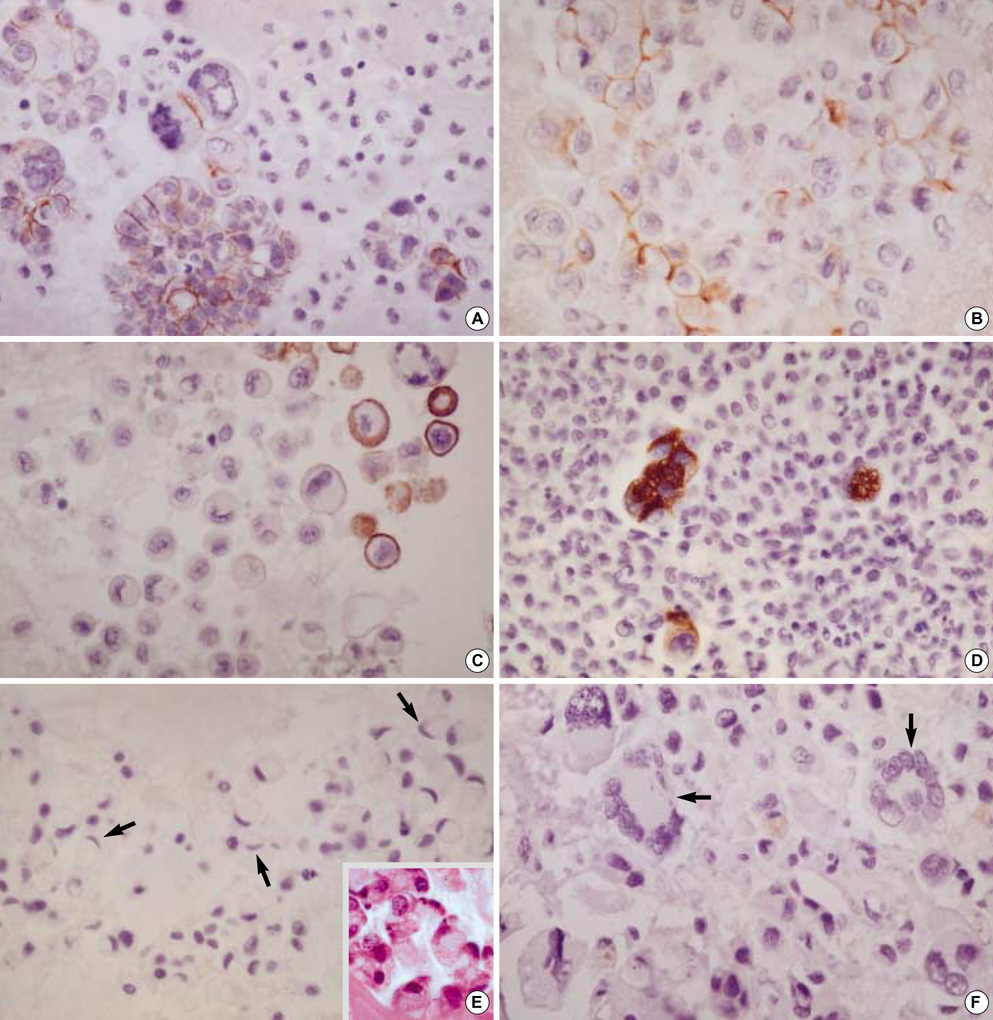
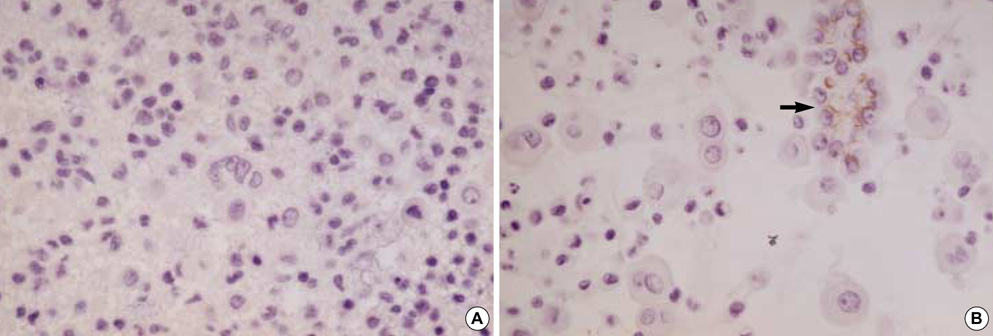
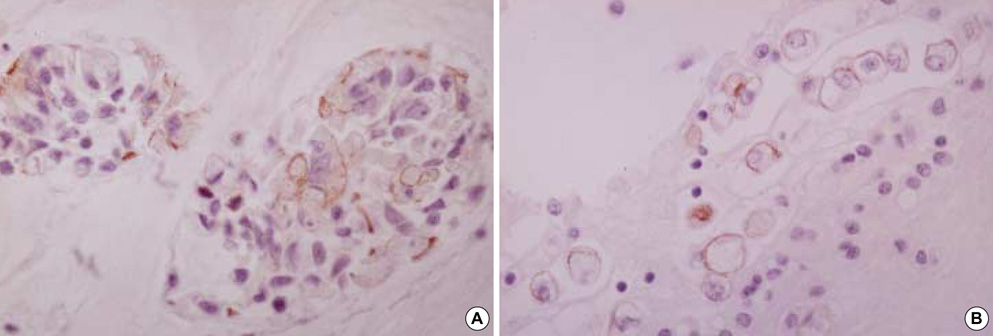
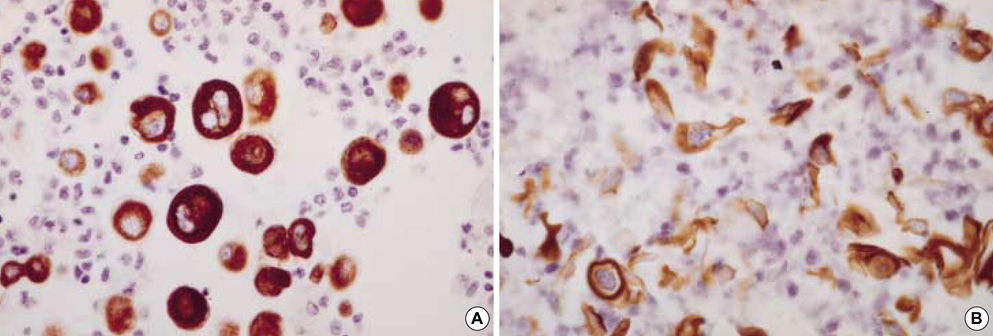
- Diagnostic utility of E-cadherin (E-CD) and cytokeratin (CK) subtype profiling in effusion cytology was investigated, employing immunocytochemistry on cellblock sections available from 211 metastatic carcinomas (MC), 6 mesotheliomas and 73 reactive mesothelial hyperplasias (MH). E-CD and monoclonal carcinoembryonic anti-gen (mCEA) stained 85% (120/141) and 65% (138/211) of MC, respectively. E-CD staining of MC was frequently heterogeneous (76/120) and absent in all anaplastic carcinomas (0/2). E-CD stained none (0/57) of MH while mCEA and epithelial membrane antigen (EMA) stained 12% (9/73) and 32% (16/32) of MH, respectively. Of 6 mesotheliomas, E-CD focally stained in 2 while mCEA stained none and EMA stained all. CK20 and CK17 stained none of MH or mesotheliomas. CK20 stained 15% of MC and CK 17 stained 22% of MC. CK5/6 and high molecular weight CK stained all mesotheliomas, 56% and 88% of MH, 26% and 39% of MC, respectively. MC showed predominant CK7+/20-expression, with the exceptions of MC from mucinous type of colon/rectum and ovary showing predominant CK20 positive. E-CD may be a useful positive marker for MC in effusion cytology, although it may focally stain in some mesotheliomas. Any positive staining for CK20 of MC suggests MC from the gastrointestinal tract or ovary among others.
Keyword
MeSH Terms
Figure
Reference
-
1. Peralta Soler A, Knudsen KA, Jaurand MC, Johnson KR, Wheelock MJ, Klein-Szanto AJ, Salazar H. The differential expression of N-cadherin and E-cadherin distinguishes pleural mesotheliomas from lung adenocarcinomas. Hum Pathol. 1995. 26:1363–1369.
Article2. Leers MP, Aarts MM, Theunissen PH. E-cadherin and calretinin: a useful combination of immunochemical markers for differentiation between mesothelioma and metastatic adenocarcinoma. Histopathology. 1998. 32:209–216.
Article3. Lim SJ, Kim GY, Kim YW, Park YK, Lee J, Yang MH, Won NH. Usefulness of E-cadherin expression in malignant effusion. Korean J Cytopathol. 1999. 10:121–126.4. Ordonez NG. Value of cytokeratin 5/6 immunostaining in distinguishing epithelial mesothelioma of the pleura from lung adenocarcinoma. Am J Surg Pathol. 1998. 22:1215–1221.5. Ordonez NG. Value of thyroid transcription factor-1, E-cadherin, BG8, WT1, and CD44S immunostaining in distinguishing epithelial pleural mesothelioma from pulmonary and nonpulmonary adenocarcinoma. Am J Surg Pathol. 2000. 24:598–606.6. Kim BH, Kwon OJ. Immunocytochemical expression of E-cadherin in cell blocks of serous effusions. Korean J Cytopathol. 2001. 12:81–88.7. Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991. 251:1451–1455.
Article8. Shimoyama Y, Hirohashi S, Hirano S, Noguchi M, Shimosato Y, Takeichi M, Abe O. Cadherin cell-adhesion molecules in human epithelial tissues and carcinomas. Cancer Res. 1989. 49:2128–2133.9. Peralta Soler A, Knudsen KA, Tecson-Miguel A, McBrearty FX, Han AC, Salazar H. Expression of E-cadherin and N-cadherin in surface epithelial-stromal tumors of the ovary distinguishes mucinous from serous and endometrioid tumors. Hum Pathol. 1997. 28:734–739.10. Simsir A, Fetsch P, Mehta D, Zakowski M, Abati A. E-cadherin, N-cadherin, and calretinin in pleural effusions: the good, the bad, the worthless. Diagn Cytopathol. 1999. 20:125–130.
Article11. Cooper D, Schermer A, Sun TT. Classification of human epithelia and their neoplasms using monoclonal antibodies to keratins: strategies, applications, and limitations. Lab Invest. 1985. 52:243–256.12. Battifora H. Sternberg SS, editor. Pleura. Diagnostic Surgical Pathology. 1999. 3rd ed. Philadelphia: Lippincott Williams & Wilkins;1117–1145.13. Clover J, Oates J, Edwards C. Anti-cytokeratin 5/6: a positive marker for epithelioid mesothelioma. Histopathology. 1997. 31:140–143.
Article14. O'Malley FP FP, Grignon DJ, Shum DT. Usefulness of immunoperoxidase staining with high-molecular-weight cytokeratin in the differential diagnosis of small-acinar lesions of the prostate gland. Virchow Arch A Pathol Anat Histopathol. 1990. 417:191–196.15. Wojno KJ, Epstein JI. The utility of basal cell-specific anti-cytokeratin antibody (34bE12) in the diagnosis of prostate cancer. A review of 228 cases. Am J Surg Pathol. 1995. 19:251–260.16. Moinfar F, Man YG, Lininger RA, Bodian C, Tavassoli FA. Use of keratin 34betaE12 as an adjunct in the diagnosis of mammary intraepithelial neoplasia-ductal type-benign and malignant intraductal proliferations. Am J Surg Pathol. 1999. 23:1048–1058.17. Miettinen M, Nobel MP, Tuma BT, Kovatich AJ. Keratin 17: Immunohistochemical mapping of its distribution in human epithelial tumors and its potential applications. Appl Immunohistochem. 1997. 5:152–159.18. Ordonez NG. Role of immunohistochemistry in distinguishing epithelial peritoneal mesotheliomas from peritoneal and ovarian serous carcinomas. Am J Surg Pathol. 1998. 22:1203–1214.19. Shimoyama Y, Hirohashi S. Cadherin intercellular adhesion molecule in hepatocellular carcinomas: loss of E-cadherin expression in an undifferentiated carcinoma. Cancer Lett. 1991. 57:131–135.
Article20. Mayer B, Johnson JP, Leitl F, Jauch KW, Heiss MM, Schildberg FW, Birchmeier W, Funke I. E-cadherin expression in primary and metastatic gastric cancer: down-regulation correlates with cellular dedifferentiation and glandular disintegration. Cancer Res. 1993. 53:1690–1695.21. Pignatelli M, Liu D, Nasim MM, Stamp GW, Hirano S, Takeichi M. Morphoregulatory activities of E-cadherin and beta-1 integrins in colorectal tumour cells. Br J Cancer. 1992. 66:629–634.
Article22. Hashimoto M, Niwa O, Nitta Y, Takeichi M, Yokoro K. Unstable expression of E-cadherin adhesion molecules in metastatic ovarian tumor cells. Jpn J Cancer Res. 1989. 80:459–463.
Article23. Bussemakers MJ, van Moorselaar RJ, Giroldi LA, Ichikawa T, Isaacs JT, Takeichi M, Debruyne FM, Schalken JA. Decreased expression of E-cadherin in the progression of rat prostatic cancer. Cancer Res. 1992. 52:2916–2922.24. Kinsella AR, Green B, Lepts GC, Hill CL, Bowie G, Taylor BA. The role of the cell-cell adhesion molecule E-cadherin in large bowel tumour cell invasion and metastasis. Br J Cancer. 1993. 67:904–909.
Article25. Ross JS, Figge HL, Bui HX, del Rosario AD, Fisher HA, Nazeer T, Jennings TA, Ingle R, Kim DN. E-cadherin expression in prostatic carcinoma biopsies: correlation with tumor grade, DNA content, pathologic stage, and Mod Pathol clinical outcome. Mod Pathol. 1994. 7:835–841.26. Tsuji K, Hirano T, Shibanuma H, Okada S, Kawate N, Konaka C, Ebihara Y, Kato H. Cytologic features based on the expression of E-cadherin and catenins in lung adenocarcinoma. Acta Cytol. 1999. 43:381–389.
Article27. Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, Lochner D, Birchmeier W. E-cadherin mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991. 113:173–185.28. Ross JS, Cheung C, Sheehan C, del Rosario AD, Bui HX, Fisher HA. E-cadherin cell-adhesion molecule expression as a diagnostic adjunct in urothelial cytology. Diagn Cytopathol. 1996. 14:310–315.
Article29. Gamallo C, Palacios J, Suarez A, Pizarro A, Navarro P, Quintanilla M, Cano A. Correlation of E-cadherin expression with differentiation grade and histological type in breast carcinoma. Am J Pathol. 1993. 142:987–993.30. Taki A, Nakatani Y, Misugi K, Yao M, Nagashima Y. Chromophobe renal cell carcinoma: An immunohistochemical study of 21 Japanese cases. Mod Pathol. 1999. 12:310–317.31. Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: A survey of 435 cases. Mod Pathol. 2000. 13:962–972.
Article32. Wang NP, Zee S, Zarbo RJ, Bacchi CE, Gown AM. Coordinate expression of cytokeratins 7 and 20 defines unique subsets of carcinomas. Appl Immunohistochem. 1995. 3:99–107.33. Ascoli V, Taccogna S, Scalzo CC, Nardi F. Utility of cytokeratin 20 in identifying the origin of metastatic carcinomas in effusions. Diagn Cytopathol. 1995. 12:303–308.
Article34. Miettinen M. Keratin 20: Immunohistochemical marker for gastrointestinal, urothelial, and Merkel cell carcinomas. Mod Pathol. 1995. 8:384–388.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunocytochemical Expression of E-cadherin in Cell Blocks of Serous Effusions
- The Expression of E-cadherin in Human and Rat Hepatic Stellate Cells: Evidence of Epithelial-Mesenchymal Transition
- Two Cases of Yersinia Pseudotuberculosis Infection with Acute Renal Failure in Pusan Province
- Cytologic Diagnosis of Malignant Pleural Effusion in Multiple Myeloma: Two Case Reports
- Usefulness of E-Cadherin Expression in Malignant Effusion