J Korean Med Sci.
2009 Jan;24(Suppl 1):S148-S155. 10.3346/jkms.2009.24.S1.S148.
Successful Renal Transplantation with Desensitization in Highly Sensitized Patients: A Single Center Experience
- Affiliations
-
- 1Division of Nephrology, Department of Internal Medicine, Kangnam St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. yangch@catholic.ac.kr
- 2Department of Laboratory Medicine, Kangnam St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Department of Surgery, Kangnam St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 1778155
- DOI: http://doi.org/10.3346/jkms.2009.24.S1.S148
Abstract
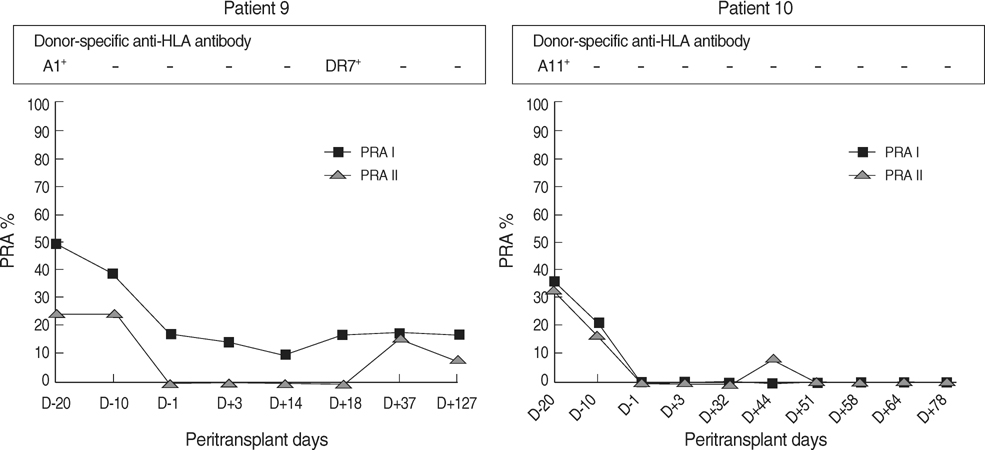
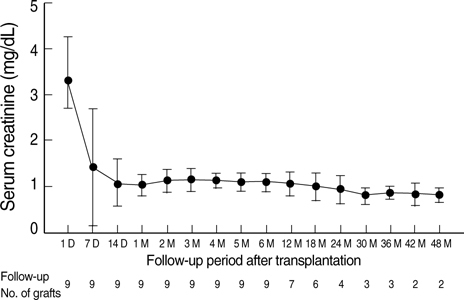
- Intravenous immunoglobulin (IVIG) and/or plasmapheresis (PP) are effective in preventing antibody-mediated rejection (AMR) of kidney allografts, but AMR is still a problem. This study reports our experience in living donor renal transplantation in highly sensitized patients. Ten patients with positive crossmatch tests or high levels of panel-reactive antibody (PRA) were included. Eight patients were desensitized with pretransplant PP and low dose IVIG, and two were additionally treated with rituximab. Allograft function, number of acute rejection (AR) episodes, protocol biopsy findings, and the presence of donor-specific antibody (DSA) were evaluated. With PP/IVIG, six out of eight patients showed good graft function without AR episodes. Protocol biopsies revealed no evidence of tissue injury or C4d deposits. Of two patients with AR, one was successfully treated with PP/IVIG, but the other lost graft function due to de novo production of DSA. Thereafter, rituximab was added to PP/IVIG in two cases. Rituximab gradually decreased PRA levels and the percentage of peripheral CD20+ cells. DSA was undetectable and protocol biopsy showed no C4d deposits. The graft function was stable and there were no AR episodes. Conclusively, desensitization using PP/IVIG with or without rituximab increases the likelihood of successful living donor renal transplantation in sensitized recipients.
Keyword
MeSH Terms
-
Adult
Antibodies, Monoclonal/therapeutic use
Antigens, CD20/biosynthesis
Female
Humans
Immunoglobulins/metabolism
Immunophenotyping
Immunosuppressive Agents/therapeutic use
Isoantibodies/chemistry
Kidney Failure, Chronic/therapy
Kidney Transplantation/*methods
Lymphocytes/metabolism
Male
Middle Aged
Retrospective Studies
Figure
Cited by 1 articles
-
Cardiovascular Diseases after Kidney Transplantation in Korea
Jong Cheol Jeong, Han Ro, Young-Hwan Hwang, Han Kyu Lee, Jongwon Ha, Curie Ahn, Jaeseok Yang
J Korean Med Sci. 2010;25(11):1589-1594. doi: 10.3346/jkms.2010.25.11.1589.
Reference
-
1. Montgomery RA, Zachary AA, Racusen LC, Leffell MS, King KE, Burdick J, Maley WR, Ratner LE. Plasmapheresis and intravenous immune globulin provides effective rescue therapy for refractory humoral rejection and allows kidneys to be successfully transplanted into cross-match-positive recipients. Transplantation. 2000. 70:887–895.
Article2. Stegall MD, Gloor J, Winters JL, Moore SB, DeGoey S. A comparison of plasmapheresis versus high-dose IVIG desensitization in renal allograft recipients with high levels of donor specific alloantibody. Am J Transplant. 2006. 6:346–351.
Article3. Glotz D, Antoine C, Julia P, Suberbielle-Boissel C, Boudjeltia S, Fraoui R, Hacen C, Duboust A, Bariety J. Desensitization and subsequent kidney transplantation of patients using intravenous immunoglobulins (IVIg). Am J Transplant. 2002. 2:758–760.
Article4. Jordan SC, Vo AA, Peng A, Toyoda M, Tyan D. Intravenous gammaglobulin (IVIG): a novel approach to improve transplant rates and outcomes in highly HLA-sensitized patients. Am J Transplant. 2006. 6:459–466.
Article5. Zachary AA, Montgomery RA, Ratner LE, Samaniego-Picota M, Haas M, Kopchaliiska D, Leffell MS. Specific and durable elimination of antibody to donor HLA antigens in renal-transplant patients. Transplantation. 2003. 76:1519–1525.
Article6. Vo AA, Toyoda M, Peng A, Bunnapradist S, Lukovsky M, Jordan SC. Effect of induction therapy protocols on transplant outcomes in crossmatch positive renal allograft recipients desensitized with IVIG. Am J Transplant. 2006. 6:2384–2390.
Article7. Schweitzer EJ, Wilson JS, Fernandez-Vina M, Fox M, Gutierrez M, Wiland A, Hunter J, Farney A, Philosophe B, Colonna J, Jarrell BE, Bartlett ST. A high panel-reactive antibody rescue protocol for crossmatch-positive live donor kidney transplants. Transplantation. 2000. 70:1531–1536.8. Jordan SC, Tyan D, Stablein D, Mcintosh M, Rose S, Vo A, Toyoda M, Davis C, Shapiro R, Adey D, Milliner D, Graff R, Steiner R, Ciancio G, Sahney S, Light J. Evaluation of intravenous immunoglobulin as an agent to lower allosensitization and improve transplantation in highly sensitized adult patients with end-stage renal disease: report of the NIH IG02 trial. J Am Soc Nephrol. 2004. 15:3256–3262.
Article9. Magee CC, Mah H, Tinckam K, Wood I, Ji F, Powelson J. Successful living donor kidney transplantation across HLA and ABO incompatibilities. Nephrol Dial Transplant. 2007. 22:602–604.
Article10. Gloor JM, DeGoey SR, Pineda AA, Moore SB, Prieto M, Nyberg SL, Larson TS, Griffen MD, Textor SC, Velosa JA, Schwab TR, Fix LA, Stegall MD. Overcoming a positive crossmatch in living-donor kidney transplantation. Am J Transplant. 2003. 3:1017–1023.
Article11. Sawada T, Fuchinoue S, Teraoka S. Successful A1-to-O ABO-incompatible kidney transplantation after a preconditioning regimen consisting of anti-CD20 monoclonal antibody infusions, splenectomy, and double-filtration plasmapheresis. Transplantation. 2002. 74:1207–1210.
Article12. Sonnenday CJ, Warren DS, Cooper M, Samaniego M, Haas M, King KE, Simpkins CE, Montgomery RA. Plasmapheresis, CMV hyperimmune globulin, and anti-CD20 allow ABO-incompatible renal transplantation without splenectomy. Am J Transplant. 2004. 4:1315–1322.
Article13. Tydén G, Kumlien G, Genberg H, Sandberg J, Lundgren T, Fehrman I. ABO incompatible kidney transplantations without splenectomy, using antigen-specific immunoadsorption and rituximab. Am J Transplant. 2005. 5:145–148.
Article14. Yang CW, Oh EJ, Lee SB, Moon IS, Kim DG, Choi BS, Park SC, Choi YJ, Park YJ, Han K. Detection of donor-specific anti-HLA class I and II antibodies using antibody monitoring system. Transplant Proc. 2006. 38:2803–2806.
Article15. Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns JP, Demetris AJ, Fishbein MC, Fogo A, Furness P, Gibson IW, Glotz D, Hayry P, Hunsickern L, Kashgarian M, Kerman R, Magil AJ, Montgomery R, Morozumi K, Nickeleit V, Randhawa P, Regele H, Seron D, Seshan S, Sund S, Trpkov K. Antibody-mediated rejection criteria-an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003. 3:708–714.16. Book BK, Agarwal A, Milgrom AB, Bearden CM, Sidner RA, Higgins NG, Pescovitz MD. New crossmatch technique eliminates interference by humanized and chimeric monoclonal antibodies. Transplant Proc. 2005. 37:640–642.
Article17. Opelz G. Collaborative transplant study-10-year report. Transplat Proc. 1992. 24:2342–2355.18. Montgomery RA, Zachary AA. Transplanting patients with a positive donor-specific crossmatch: A single center's perspective. Pediatr Transplantation. 2004. 8:535–542.
Article19. Kotb M, Russell WC, Hathaway DK, Gaber LW, Gaber AO. The use of positive B cell flow cytometry crossmatch in predicting rejection among renal transplant recipients. Clin Transplant. 1999. 13:83–89.
Article20. Lazda VA. Identification of patients at risks for inferior renal allograft outcome by a strongly positive B cell flow cytometry crossmatch. Transplantation. 1994. 57:964–969.21. Tanabe K. Japanese experience of ABO-incompatible living kidney transplantation. Transplantation. 2007. 84:S4–S7.
Article22. Vieira CA, Agarwal A, Book BK, Sidner RA, Bearden CM, Gebel HM, Roggero AL, Fineberg NS, Taber T, Kraus MA, Pescovitz MD. Rituximab for reduction of anti-HLA antibodies in patients awaiting renal transplantation: 1. safety, pharmacodynamics, and pharmacokinetics. Transplantation. 2004. 77:542–548.23. Genberg H, Hansson A, Wernerson A, Wennberg L, Tydén G. Pharmacodynamics of rituximab in kidney transplantation. Transplantation. 2007. 84:S33–S36.
Article24. Matignon M, Tagnaouti M, Audard V, Dahan K, Lang P, Grimbert P. Failure of anti-CD20 monoclonal antibody therapy to prevent antibody-mediated rejection in three crossmatch-positive renal transplant recipients. Transplant Proc. 2007. 39:2565–2567.
Article25. Beimler JH, Susal C, Zeier M. Desensitization strategies enabling successful renal transplantation in highly sensitized patients. Clin Transplant. 2006. 20 Suppl 17:7–12.
Article26. Choi BS, Shin MJ, Shin SJ, Kim YS, Choi YJ, Kim YS, Moon IS, Kim SY, Koh YB, Bang BK, Yang CW. Clinical significance of an early protocol biopsy in living-donor renal transplantation: ten-year experience at a single center. Am J Transplant. 2005. 5:1354–1360.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Renal Transplantation in Highly Sensitized Recipients
- A Case of Successful Third Renal Transplantation Using Rituximab (anti-CD20 Monoclonal Antibody) in a Highly Sensitized Patient
- Desensitization in HLA Incompatible Transplantation
- Long-term outcomes of emergency ABO-incompatible living donor liver transplantation using a modified desensitization protocol for highly sensitized patients with acute liver failure: A case report
- Successful desensitization and transplantation of kidney transplant recipients with donor-specific antibodies