Polyphenol (-)-Epigallocatechin Gallate during Ischemia Limits Infarct Size Via Mitochondrial K(ATP) Channel Activation in Isolated Rat Hearts
- Affiliations
-
- 1Department of Physiology1, School of Medicine, Keimyung University, Daegu, Korea.
- 2Department of Anesthesiology, Pureun Hospital, Daegu, Korea. weonjo@dsmc.or.kr
- 3Institute of Cardiovascular Research, Pusan National University Yangsan Hospital, Yangsan, Korea.
- 4Department of Anesthesiology and Pain Medicine, College of Medicine, Yeungnam University, Daegu, Korea.
- 5Department of Anesthesiology, University of North Carolina, Chapel Hill, North Carolina, USA.
- KMID: 1778032
- DOI: http://doi.org/10.3346/jkms.2010.25.3.380
Abstract
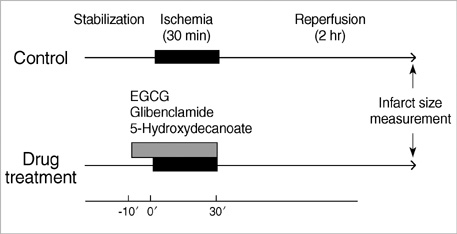
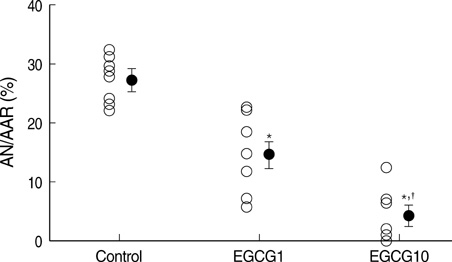
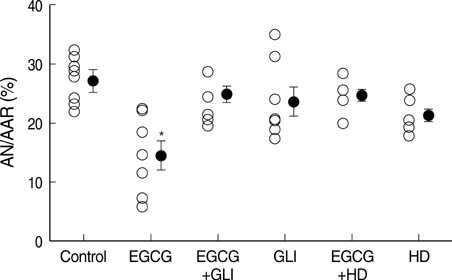
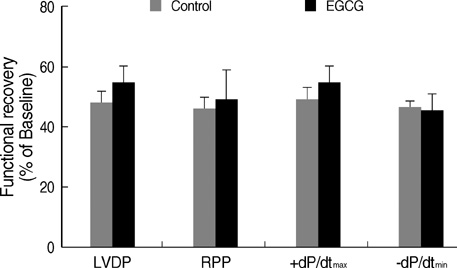
- Polyphenol (-)-epigallocatechin gallate (EGCG), the most abundant catechin of green tea, appears to attenuate myocardial ischemia/reperfusion injury. We investigated the involvement of ATP-sensitive potassium (K(ATP)) channels in EGCG-induced cardioprotection. Isolated rat hearts were subjected to 30 min of regional ischemia and 2 hr of reperfusion. EGCG was perfused for 40 min, from 10 min before to the end of index ischemia. A nonselective K(ATP) channel blocker glibenclamide (GLI) and a selective mitochondrial K(ATP) (mK(ATP)) channel blocker 5-hydroxydecanoate (HD) were perfused in EGCG-treated hearts. There were no differences in coronary flow and cardiodynamics including heart rate, left ventricular developed pressure, rate-pressure product, +dP/dt(max), and -dP/dt(min) throughout the experiments among groups. EGCG-treatment significantly reduced myocardial infarction (14.5+/-2.5% in EGCG 1 micrometer and 4.0+/-1.7% in EGCG 10 micrometer, P<0.001 vs. control 27.2+/-1.4%). This anti-infarct effect was totally abrogated by 10 micrometer GLI (24.6+/-1.5%, P<0.001 vs. EGCG). Similarly, 100 micrometer HD also aborted the anti-infarct effect of EGCG (24.1+/-1.2%, P<0.001 vs. EGCG ). These data support a role for the K(ATP) channels in EGCG-induced cardioprotection. The mK(ATP) channels play a crucial role in the cardioprotection by EGCG.
Keyword
MeSH Terms
-
Animals
Anti-Arrhythmia Agents/pharmacology
Antioxidants/*pharmacology
Catechin/*analogs & derivatives/pharmacology
Decanoic Acids/pharmacology
Glyburide/pharmacology
Heart/*drug effects/physiology/physiopathology
Hemodynamics
Humans
Hydroxy Acids/pharmacology
KATP Channels/*metabolism
Male
Mitochondria, Heart/*drug effects/metabolism
Myocardial Infarction/*pathology
Myocardial Ischemia/*pathology
Potassium Channel Blockers/pharmacology
Rats
Rats, Wistar
Anti-Arrhythmia Agents
Antioxidants
Decanoic Acids
Hydroxy Acids
KATP Channels
Potassium Channel Blockers
Glyburide
Catechin
Figure
Cited by 3 articles
-
Activated Protein C Protects Myocardium Via Activation of Anti-apoptotic Pathways of Survival in Ischemia-reperfused Rat Heart
Jia-Wang Ding, Xiao-Hong Tong, Jun Yang, Zhao-Qi Liu, Yan Zhang, Jian Yang, Song Li, Li Li
J Korean Med Sci. 2010;25(11):1609-1615. doi: 10.3346/jkms.2010.25.11.1609.Morphine-induced postconditioning modulates mitochondrial permeability transition pore opening via delta-1 opioid receptors activation in isolated rat hearts
June Hong Kim, Kook Jin Chun, Yong Hyun Park, Jun Kim, Jeong Su Kim, Young Ho Jang, Mi Young Lee, Jae Hong Park
Korean J Anesthesiol. 2011;61(1):69-74. doi: 10.4097/kjae.2011.61.1.69.The Protective Effect of Epigallocatechin-3 Gallate on Ischemia/Reperfusion Injury in Isolated Rat Hearts: An
ex vivo Approach
Cheng Shi Piao, Do-Sung Kim, Ki-Chan Ha, Hyung-Ryong Kim, Han-Jung Chae, Soo-Wan Chae
Korean J Physiol Pharmacol. 2011;15(5):259-266. doi: 10.4196/kjpp.2011.15.5.259.
Reference
-
1. Sano J, Inami S, Seimiya K, Ohba T, Sakai S, Takano T, Mizuno K. Effects of green tea intake on the development of coronary artery disease. Circ J. 2004. 68:665–670.
Article2. Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Cirt Rev Food Sci Nutr. 2003. 43:89–143.
Article3. Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, Tsubono Y, Tsuji I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA. 2006. 296:1255–1265.4. Aneja R, Hake PW, Burroughs TJ, Denenberg AG, Wong HR, Zingarelli B. Epigallocatechin, a green tea polyphenol, attenuates myocardial ischemia reperfusion injury in rats. Mol Med. 2004. 10:55–62.
Article5. Townsend PA, Scarabelli TM, Pasini E, Gitti G, Menegazzi M, Suzuki H, Knight RA, Latchman DS, Stephanou A. Epigallocatechin-3-gallate inhibits STAT-1 activation and protects cardiac myocytes from ischemia/reperfusion-induced apoptosis. FASEB J. 2004. 18:1621–1623.6. Tani M. Mechanisms of Ca2+ overload in reperfused ischemic myocardium. Annu Rev Physiol. 1990. 52:543–559.7. Ylitalo KV, Ala-Rämi A, Liimatta EV, Peuhkurinen KJ, Hassinen IE. Intracellular free calcium and mitochondrial membrane potential in ischemia/reperfusion and preconditioning. J Mol Cell Cardiol. 2000. 32:1223–1238.
Article8. Garlid KD, Paucek P, Yarov-Yarovoy V, Murray HN, Darbenzio RB, D'Alonzo AJ, Lodge NJ, Smith MA, Grover GJ. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ Res. 1997. 81:1072–1082.9. O'Rourke B. Myocardial K (ATP) channels in preconditioning. Circ Res. 2000. 87:845–855.10. Grover GJ, D'Alonzo AJ, Garlid KD, Bajgar R, Lodge NJ, Sleph PG, Darbenzio RB, Hess TA, Smith MA, Paucek P, Atwal KS. Pharmacologic characterization of BMS-191095, a mitochondrial KATP opener with no peripheral vasodilator or cardiac action potential shortening activity. J Pharmacol Exp Ther. 2001. 297:1184–1192.11. Bopassa JC, Ferrera R, Gateau-Roesch O, Couture-Lepetit E, Ovize M. PI3-kinase regulates the mitochondrial transition pore in controlled reperfusion and postconditioning. Cardiovasc Res. 2006. 69:178–185.12. Burley DS, Baxter GF. B-type natriuretic peptide at early reperfusion limits infarct size in the rat isolated heart. Basic Res Cardiol. 2007. 102:529–541.
Article13. Jang Y, Xi J, Wang H, Mueller RA, Norfleet EA, Xu Z. Postconditioning prevents reperfusion injury by activating delta-opioid receptors. Anesthesiology. 2008. 108:243–250.14. Baek WK, Jang BC, Lim JH, Kwon TK, Lee HY, Cho CH, Kim DK, Shin DH, Park JG, Lim JG, Bae JH, Yoo SK, Park WK, Song DK. Inhibitory modulation of ATP-sensitive potassium channels by gallate-ester moiety of (-)-epigallocatechin-3-gallate. Biochem Pharmacol. 2005. 70:1560–1567.
Article15. Shinohara T, Takahashi N, Kohno H, Yamanaka K, Ooie T, Wakisaka O, Murozono Y, Taniguchi Y, Torigoe Y, Hara M, Shimada T, Saikawa T, Yoshimatsu H. Mitochondria are targets for geranylgeranylacetone-induced cardioprotection against ischemia-reperfusion in the rat heart. Am J Physiol Heart Circ Physiol. 2007. 293:H1892–H1899.
Article16. Chow HH, Hakim IA, Vining DR, Crowell JA, Ranger-Moore J, Chew WM, Celaya CA, Rodney SR, Hara Y, Alberts DS. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clin Cancer Res. 2005. 11:4627–4633.
Article17. Liu H, Wang L, Eaton M, Schaefer S. Sevoflurane preconditioning limits intracellular/mitochondrial Ca2+ in ischemic newborn myocardium. Anesth Analg. 2005. 101:349–355.18. Tani M, Neely JR. Role of intracellular Na+ and Ca2+ overload and depressed recovery of ventricular function of reperfused ischemic rat hearts. Possible involvement of H+-Na+ and Na+-Ca2+ exchange. Circ Res. 1989. 65:1045–1056.19. Jin JY, Park SH, Bae JH, Cho HC, Lim JG, Park WS, Han J, Lee JH, Song DK. Uncoupling by (-)-epigallocatechin-3-gallate of ATP-sensitive potassium channels from phosphatidylinositol polyphosphates and ATP. Pharmacol Res. 2007. 56:237–247.
Article20. Wang L, Cherednichenko G, Hernandez L, Halow J, Camacho SA, Figueredo V, Schaefer S. Preconditioning limits mitochondrial Ca2+ during ischemia in rat hearts: role of KATP channels. Am J Physiol Heart Circ Physiol. 2001. 280:H2321–H2328.21. Ferrari R. The role of mitochondria in ischemic heart disease. J Cardiovasc Pharmacol. 1996. 28:Suppl 1. S1–S10.
Article22. Holmuhamedov EL, Jovanović S, Dzeja PP, Jovanović A, Terzic A. Mitochondrial ATP-sensitive K+ channels modulate cardiac mitochondrial function. Am J Physiol. 1998. 275:H1567–H1576.23. Steenbergen C, Fralix TA, Murphy E. Role of increased cytosolic free calcium concentration in myocardial ischemic injury. Basic Res Cardiol. 1993. 88:456–470.
Article24. Meissner A, Morgan JP. Contractile dysfunction and abnormal Ca2+ modulation during postischemic reperfusion in rat heart. Am J Physiol. 1995. 268:H100–H111.25. Lochner A, Genade S, Moolman JA. Ischemic preconditioning: infarct size is a more reliable endpoint than functional recovery. Basic Res Cardiol. 2003. 98:337–346.
Article26. Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res. 2002. 55:534–543.
Article27. Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ Res. 2003. 93:292–301.
Article28. Krolikowski JG, Bienengraeber M, Weihrauch D, Warltier DC, Kersten JR, Pagel PS. Inhibition of mitochondrial permeability transition enhances isoflurane-induced cardioprotection during early reperfusion: the role of mitochondrial KATP channels. Anesth Analg. 2005. 101:1590–1596.
Article29. Piriou V, Chiari P, Gateau-Roesch O, Argaud L, Muntean D, Salles D, Loufouat J, Gueugniaud PY, Lehot JJ, Ovize M. Desflurane-induced preconditioning alters calcium-induced mitochondrial permeability transition. Anesthesiology. 2004. 100:581–588.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Polyphenol (-)-epigallocatechin gallate targeting myocardial reperfusion limits infarct size and improves cardiac function
- Polyphenol (-)-epigallocatechin gallate-induced cardioprotection may attenuate ischemia-reperfusion injury through adenosine receptor activation: a preliminary study
- The Protective Effect of Epigallocatechin-3 Gallate on Ischemia/Reperfusion Injury in Isolated Rat Hearts: An ex vivo Approach
- Role of adenosine in the cardioprotective effect of ischemic preconditioning in the isolated rabbit hearts
- The Myocardial Protective Effects of Desflurane on Global Ischemia in Isolated Rat Heart via KATP Channel Activation