J Korean Med Sci.
2013 May;28(5):750-754. 10.3346/jkms.2013.28.5.750.
Clinical Usefulness of Plasma Chromogranin A in Pancreatic Neuroendocrine Neoplasm
- Affiliations
-
- 1Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea. jkryu@snu.ac.kr
- KMID: 1777564
- DOI: http://doi.org/10.3346/jkms.2013.28.5.750
Abstract
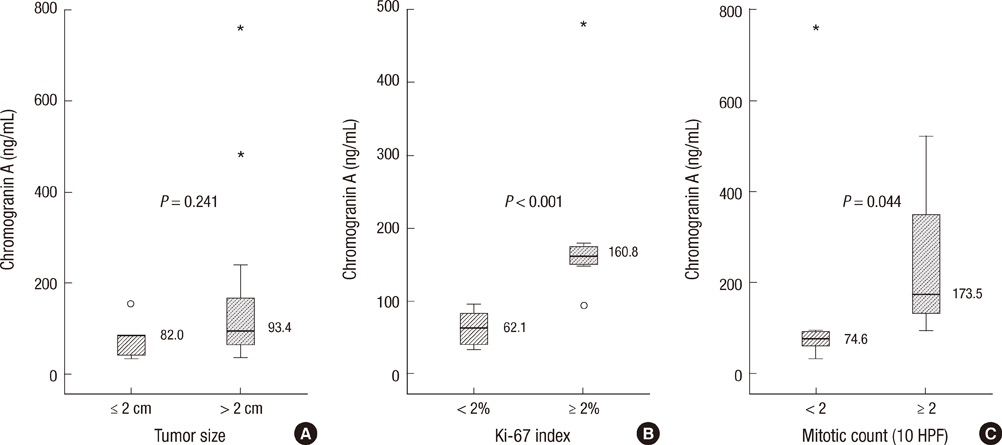
- Chromogranin A (CgA) is widely used as an immunohistochemical marker of neuroendocrine neoplasms and has been measurable in plasma of patients. We assessed the clinical role of plasma CgA in diagnosing pancreatic neuroendocrine neoplasm (PNEN). CgA was checked in 44 patients with pancreatic mass who underwent surgical resection from 2009 through 2011. The cutoff value for diagnosing PNEN and the relationships between CgA and clinicopathologic variables were analyzed. Twenty-six patients were PNENs and 18 patients were other pancreatic disorders. ROC analysis showed a cutoff of 60.7 ng/mL with 77% sensitivity and 56% specificity, and the area under the curve (AUC) was 0.679. Among PNEN group, the sensitivity and specificity of diagnosing metastasis were 100% and 90% respectively when CgA cutoff was 156.5 ng/mL. The AUC was 0.958. High Ki-67 index (160.8 vs 62.1 ng/mL, P = 0.001) and mitotic count (173.5 vs 74.6 ng/mL, P = 0.044) were significantly correlated with plasma CgA, but the tumor size was not. In conclusion, CgA has a little value in diagnosing PNEN. However, the high level of CgA (more than 156.5 ng/mL) can predict the metastasis. Also, plasma CgA level correlates with Ki-67 index and mitotic count which represents prognosis of PNENs.
MeSH Terms
Figure
Cited by 1 articles
-
Diagnosis of Pancreatic Neuroendocrine Tumors
Dong Wook Lee, Michelle Kang Kim, Ho Gak Kim
Clin Endosc. 2017;50(6):537-545. doi: 10.5946/ce.2017.131.
Reference
-
1. Mulkeen AL, Yoo PS, Cha C. Less common neoplasms of the pancreas. World J Gastroenterol. 2006. 12:3180–3185.2. Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008. 26:3063–3072.3. Halfdanarson TR, Rubin J, Farnell MB, Grant CS, Petersen GM. Pancreatic endocrine neoplasms: epidemiology and prognosis of pancreatic endocrine tumors. Endocr Relat Cancer. 2008. 15:409–427.4. Cheema A, Weber J, Strosberg JR. Incidental detection of pancreatic neuroendocrine tumors: an analysis of incidence and outcomes. Ann Surg Oncol. 2012. 19:2932–2936.5. Furukawa T, Tsukamoto Y, Naitoh Y, Mitake M, Hirooka Y, Hayakawa T. Differential diagnosis of pancreatic diseases with an intraductal ultrasound system. Gastrointest Endosc. 1994. 40:213–219.6. Sakamoto H, Kitano M, Kamata K, El-Masry M, Kudo M. Diagnosis of pancreatic tumors by endoscopic ultrasonography. World J Radiol. 2010. 2:122–134.7. Eriksson B, Oberg K. Neuroendocrine tumours of the pancreas. Br J Surg. 2000. 87:129–131.8. Milan SA, Yeo CJ. Neuroendocrine tumors of the pancreas. Curr Opin Oncol. 2012. 24:46–55.9. Paik WH, Yoon YB, Lee SH, Park JK, Woo SM, Yang KY, Seo JK, Ryu JK, Kim YT. Pancreatic endocrine tumors: clinical manifestations and predictive factors associated with survival. Korean J Gastroenterol. 2008. 52:171–178.10. Deftos LJ. Chromogranin A: its role in endocrine function and as an endocrine and neuroendocrine tumor marker. Endocr Rev. 1991. 12:181–187.11. O'Connor DT, Deftos LJ. Secretion of chromogranin A by peptide-producing endocrine neoplasms. N Engl J Med. 1986. 314:1145–1151.12. Sobol RE, Memoli V, Deftos LJ. Hormone-negative, chromogranin Apositive endocrine tumors. N Engl J Med. 1989. 320:444–447.13. Nobels FR, Kwekkeboom DJ, Coopmans W, Schoenmakers CH, Lindemans J, De Herder WW, Krenning EP, Bouillon R, Lamberts SW. Chromogranin A as serum marker for neuroendocrine neoplasia: comparison with neuron-specific enolase and the alpha-subunit of glycoprotein hormones. J Clin Endocrinol Metab. 1997. 82:2622–2628.14. Zatelli MC, Torta M, Leon A, Ambrosio MR, Gion M, Tomassetti P, De Braud F, Delle Fave G, Dogliotti L, degli Uberti EC. Chromogranin A as a marker of neuroendocrine neoplasia: an Italian Multicenter Study. Endocr Relat Cancer. 2007. 14:473–482.15. Baudin E, Bidart JM, Bachelot A, Ducreux M, Elias D, Ruffié P, Schlumberger M. Impact of chromogranin A measurement in the work-up of neuroendocrine tumors. Ann Oncol. 2001. 12:S79–S82.16. Rindi G, Klöppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, Erikssson B, Falchetti A, Falconi M, Komminoth P, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006. 449:395–401.17. Campana D, Nori F, Piscitelli L, Morselli-Labate AM, Pezzilli R, Corinaldesi R, Tomassetti P. Chromogranin A: is it a useful marker of neuroendocrine tumors? J Clin Oncol. 2007. 25:1967–1973.18. Ferolla P, Faggiano A, Mansueto G, Avenia N, Cantelmi MG, Giovenali P, Del Basso De Caro ML, Milone F, Scarpelli G, Masone S, et al. The biological characterization of neuroendocrine tumors: the role of neuroendocrine markers. J Endocrinol Invest. 2008. 31:277–286.19. Stivanello M, Berruti A, Torta M, Termine A, Tampellini M, Gorzegno G, Angeli A, Dogliotti L. Circulating chromogranin A in the assessment of patients with neuroendocrine tumours: a single institution experience. Ann Oncol. 2001. 12:S73–S77.20. Tomassetti P, Migliori M, Simoni P, Casadei R, De Iasio R, Corinaldesi R, Gullo L. Diagnostic value of plasma chromogranin A in neuroendocrine tumours. Eur J Gastroenterol Hepatol. 2001. 13:55–58.21. Syversen U, Ramstad H, Gamme K, Qvigstad G, Falkmer S, Waldum HL. Clinical significance of elevated serum chromogranin A levels. Scand J Gastroenterol. 2004. 39:969–973.22. Nobels FR, Kwekkeboom DJ, Bouillon R, Lamberts SW. Chromogranin A: its clinical value as marker of neuroendocrine tumours. Eur J Clin Invest. 1998. 28:431–440.23. Peracchi M, Conte D, Gebbia C, Penati C, Pizzinelli S, Arosio M, Corbetta S, Spada A. Plasma chromogranin A in patients with sporadic gastro-entero-pancreatic neuroendocrine tumors or multiple endocrine neoplasia type 1. Eur J Endocrinol. 2003. 148:39–43.24. Tomassetti P, Campana D, Piscitelli L, Casadei R, Santini D, Nori F, Morselli-Labate AM, Pezzilli R, Corinaldesi R. Endocrine pancreatic tumors: factors correlated with survival. Ann Oncol. 2005. 16:1806–1810.25. Panzuto F, Boninsegna L, Fazio N, Campana D, Pia Brizzi M, Capurso G, Scarpa A, De Braud F, Dogliotti L, Tomassetti P, et al. Metastatic and locally advanced pancreatic endocrine carcinomas: analysis of factors associated with disease progression. J Clin Oncol. 2011. 29:2372–2377.26. Hirschowitz BI, Worthington J, Mohnen J, Haber M. Chromogranin A in patients with acid hypersecretion and/or hypergastrinaemia. Aliment Pharmacol Ther. 2007. 26:869–878.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Update of Pathology of the Pancreatic Neuroendocrine Tumor
- Pancreatic Collision Tumor of Desmoid-Type Fibromatosis and Mucinous Cystic Neoplasm: A Case Report
- Diagnosis of Pancreatic Neuroendocrine Tumors
- Pancreatic Neuroendocrine Tumor Presenting as Acute Pancreatitis
- Usefulness of micro forceps biopsy for cystic degenerated pancreatic neuroendocrine neoplasm