Korean J Radiol.
2009 Aug;10(4):366-376. 10.3348/kjr.2009.10.4.366.
Percutaneous Radiofrequency Ablation of Hepatocellular Carcinomas Adjacent to the Gallbladder with Internally Cooled Electrodes: Assessment of Safety and Therapeutic Efficacy
- Affiliations
-
- 1Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea. rhimhc@skku.edu
- 2Department of Radiology, Cheonan Hospital, Soonchunhyang University School of Medicine, Cheonan 330-721, Korea.
- 3Department of Radiology, Dankook University Hospital, Cheonan 330-715, Korea.
- KMID: 1777265
- DOI: http://doi.org/10.3348/kjr.2009.10.4.366
Abstract
OBJECTIVE
The objective of this study was to evaluate the safety and therapeutic efficacy of percutaneous radiofrequency (RF) ablation for the treatment of hepatocellular carcinomas (HCCs) adjacent to the gallbladder with the use of internally cooled electrodes.
MATERIALS AND METHODS
We retrospectively assessed 45 patients with 46 HCCs (mean size, 2.2 cm) adjacent to the gallbladder (< or =1.0 cm) treated with RF ablation using an internally cooled electrode system. An electrode was inserted into the tumor either parallel (n = 38) or perpendicular (n = 8) to the gallbladder wall. The safety and therapeutic efficacy of the procedures were assessed with clinical and imaging follow-up examinations. Follow-up with the use of CT ranged from four to 45 months (mean, 19 months). The association between variables (electrode direction, electrode type, tumor size, tumor location, lobar location) and the presence of a residual tumor or local tumor progression was also analyzed.
RESULTS
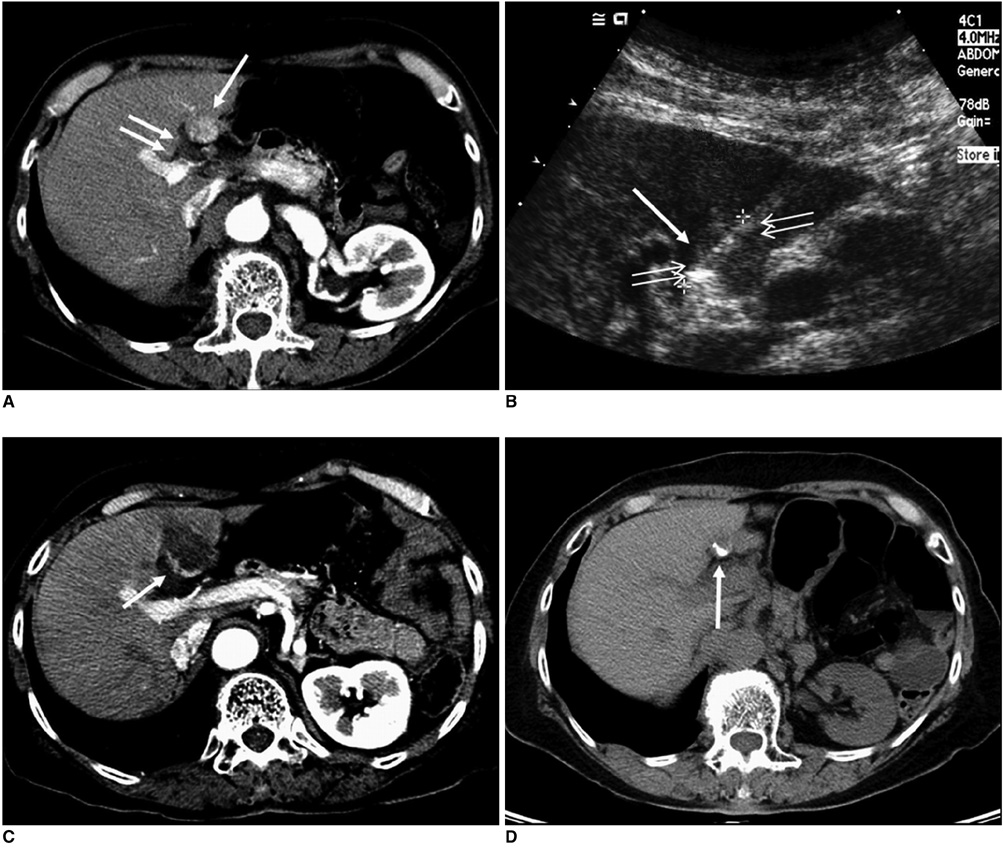
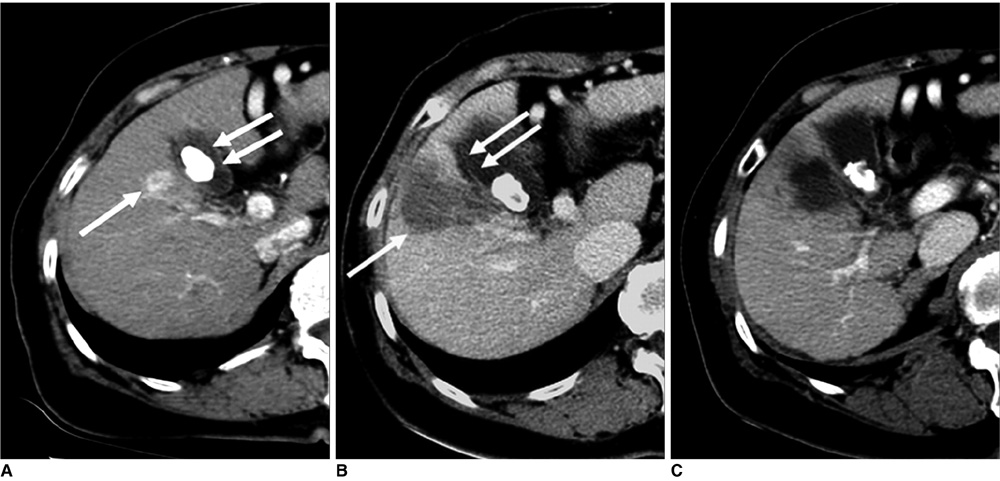
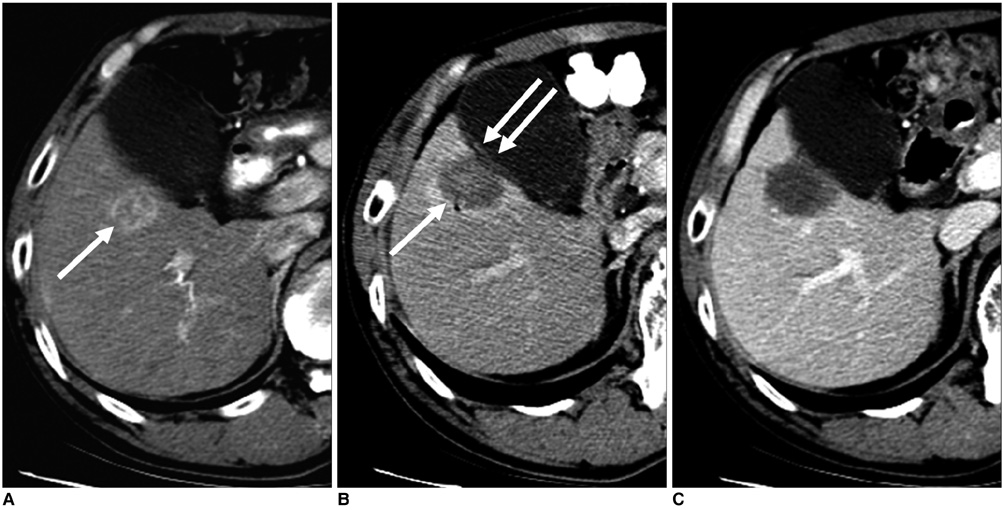
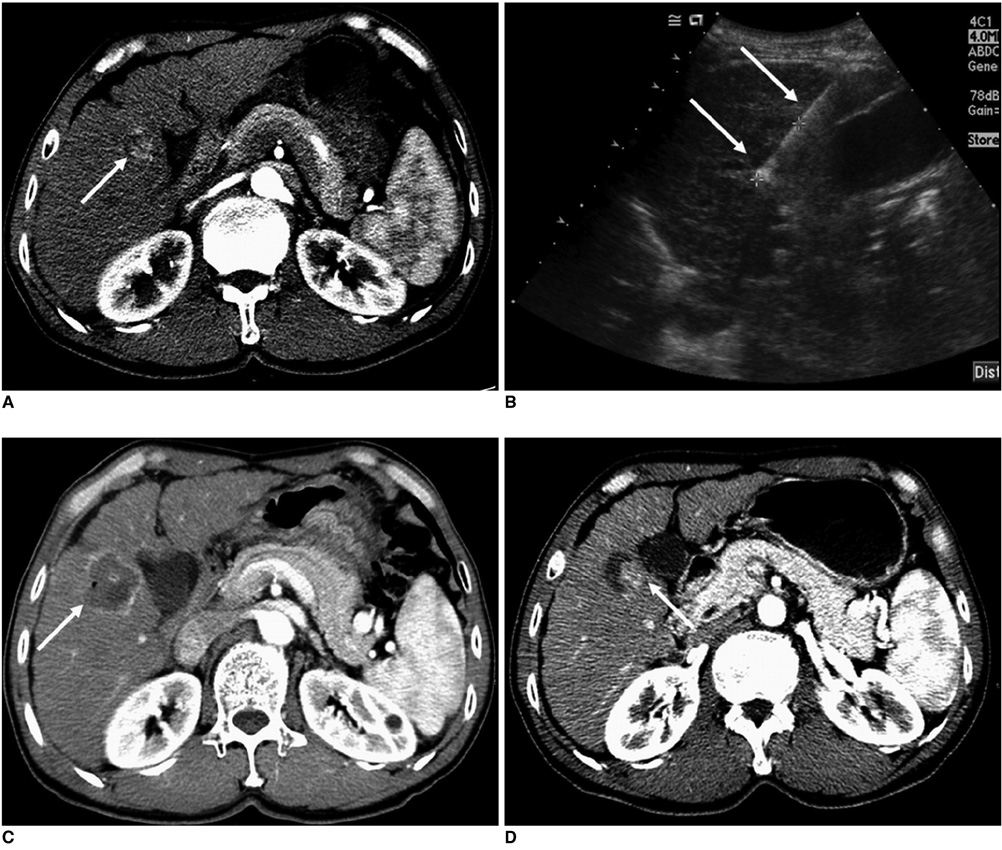
There were no major complications and minor complications were noted in three patients (7%) including one case of vasovagal syncope and two cases of bilomas. Wall thickening of the gallbladder adjacent to the RF ablation zone was noted in 14 patients (41%) as determined on immediate follow-up CT imaging. Wall thickening showed complete disappearance on subsequent follow-up CT imaging. The primary technique effectiveness rate was 96% (44/46) based on one-month follow-up CT imaging. Local tumor progression was noted in six (14%) of 44 completely ablated tumors during the follow-up period. The direction of electrode insertion (perpendicular), tumor size (> or =3 cm) and tumor location (a tumor that abutted the gallbladder) were associated with an increased risk of early incomplete treatment. No variable was significantly associated with local tumor progression.
CONCLUSION
Percutaneous RF ablation of HCCs adjacent to the gallbladder using an internally cooled electrode is a safe and effective treatment. Significant risk factors that lead to early incomplete treatment include tumor size, tumor location and electrode direction.
MeSH Terms
Figure
Cited by 2 articles
-
Percutaneous Radiofrequency Ablation with Multiple Electrodes for Medium-Sized Hepatocellular Carcinomas
Jung Lee, Jeong Min Lee, Jung-Hwan Yoon, Jae Young Lee, Se Hyung Kim, Jeong Eun Lee, Joon Koo Han, Byung Ihn Choi
Korean J Radiol. 2012;13(1):34-43. doi: 10.3348/kjr.2012.13.1.34.Evaluation of the
In Vivo Efficiency and Safety of Hepatic Radiofrequency Ablation Using a 15-G Octopus® in Pig Liver
Eun Sun Lee, Jeong Min Lee, Kyung Won Kim, In Joon Lee, Joon Koo Han, Byung Ihn Choi
Korean J Radiol. 2013;14(2):194-201. doi: 10.3348/kjr.2013.14.2.194.
Reference
-
1. Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005. 129:122–130.2. Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003. 228:235–240.3. Shibata T, Iimuro Y, Yamamoto Y, Maetani Y, Ametani F, Itoh K, et al. Small hepatocellular carcinoma: comparison of radiofrequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002. 223:331–337.4. Catalano O, Esposito M, Nunziata A, Siani A. Multiphase helical CT findings after percutaneous ablation procedures for hepatocellular carcinoma. Abdom Imaging. 2000. 25:607–614.5. Ikeda M, Okada S, Ueno H, Okusaka T, Kuriyama H. Radiofrequency ablation and percutaneous ethanol injection in patients with small hepatocellular carcinoma: a comparative study. Jpn J Clin Oncol. 2001. 31:322–326.6. Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006. 243:321–328.7. Hong SN, Lee SY, Choi MS, Lee JH, Koh KC, Paik SW, et al. Comparing the outcomes of radiofrequency ablation and surgery in patients with a single small hepatocellular carcinoma and well-preserved hepatic function. J Clin Gastroenterol. 2005. 39:247–252.8. Patterson EJ, Scudamore CH, Owen DA, Nagy AG, Buczkowski AK. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg. 1998. 227:559–565.9. Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999. 210:655–661.10. Akahane M, Koga H, Kato N, Yamada H, Uozumi K, Tateishi R, et al. Complications of percutaneous radiofrequency ablation for hepatocellular carcinoma: imaging spectrum and management. Radiographics. 2005. 25:S57–S68.11. Chen MH, Wei Y, Yan K, Gao W, Dai Y, Huo L, et al. Treatment strategy to optimize radiofrequency ablation for liver malignancies. J Vasc Interv Radiol. 2006. 17:671–683.12. Chen MH, Yang W, Yan K, Hou YB, Dai Y, Gao W, et al. Radiofrequency ablation of problematically located hepatocellular carcinoma: tailored approach. Abdom Imaging. 2008. 33:428–436.13. Chopra S, Dodd GD 3rd, Chanin MP, Chintapalli KN. Radiofrequency ablation of hepatic tumors adjacent to the gallbladder: feasibility and safety. AJR Am J Roentgenol. 2003. 180:697–701.14. Teratani T, Yoshida H, Shiina S, Obi S, Sato S, Tateishi R, et al. Radiofrequency ablation for hepatocellular carcinoma in socalled high-risk locations. Hepatology. 2006. 43:1101–1108.15. Ng KK, Lam CM, Poon RT, Shek TW, Yu WC, To JY, et al. Porcine liver: morphologic characteristics and cell viability at experimental radiofrequency ablation with internally cooled electrodes. Radiology. 2005. 235:478–486.16. Lim HK. Radiofrequency thermal ablation of hepatocellular carcinomas. Korean J Radiol. 2000. 1:175–184.17. Lim HK, Choi D, Lee WJ, Kim SH, Lee SJ, Jang HJ, et al. Hepatocellular carcinoma treated with percutaneous radiofrequency ablation: evaluation with follow-up multiphase helical CT. Radiology. 2001. 221:447–454.18. Choi D, Lim HK, Kim MJ, Kim SH, Lee WJ, Kim SH, et al. Therapeutic efficacy and safety of percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the gastrointestinal tract. AJR Am J Roentgenol. 2004. 183:1417–1424.19. Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD 3rd, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2005. 16:765–778.20. Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, et al. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics. 2003. 23:123–134.21. Lee J, Rhim H, Jeon YH, Lim HK, Lee WJ, Choi D, et al. Radiofrequency ablation of liver adjacent to body of gallbladder: histopathologic changes of gallbladder wall in a pig model. AJR Am J Roentgenol. 2008. 190:418–425.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Enhanced radiofrequency ablation for recurrent hepatocellular carcinoma post-transarterial chemoembolization: a prospective study utilizing twin internally cooled-perfusion electrodes
- Percutaneous Radiofrequency Ablation for the Hepatocellular Carcinoma Abutting the Diaphragm: Assessment of Safety and Therapeutic Efficacy
- Percutaneous cryoablation for hepatocellular carcinoma
- Percutaneous Radiofrequency Ablation with Multiple Electrodes for Medium-Sized Hepatocellular Carcinomas
- Bipolar Radiofrequency Ablation Using Dual Internally Cooled Wet Electrodes: Experimental Study in Ex Vivo Bovine Liver