Yonsei Med J.
2009 Dec;50(6):825-831. 10.3349/ymj.2009.50.6.825.
Effects of Polycaprolactone-Tricalcium Phosphate, Recombinant Human Bone Morphogenetic Protein-2 and Dog Mesenchymal Stem Cells on Bone Formation: Pilot Study in Dogs
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, Ewha Womans University School of Medicine, Seoul, Korea.
- 2Department of Advanced Prosthodontics, Institute for Clinical Dental Research, Korea University College of Medicine, Seoul, Korea. swshin@korea.ac.kr
- KMID: 1777098
- DOI: http://doi.org/10.3349/ymj.2009.50.6.825
Abstract
- PURPOSE
The aim of this study was to evaluate the survival, proliferation, and bone formation of dog mesenchymal stem cells (dMSCs) in the graft material by using Polycaprolactone-tricalcium phosphate (PCL-TCP), auto-fibrin glue (AFG), recombinant human bone morphogenetic protein-2 (rhBMP-2), and dMSCs after a transplantation to the scapula of adult beagle dogs.
MATERIALS AND METHODS
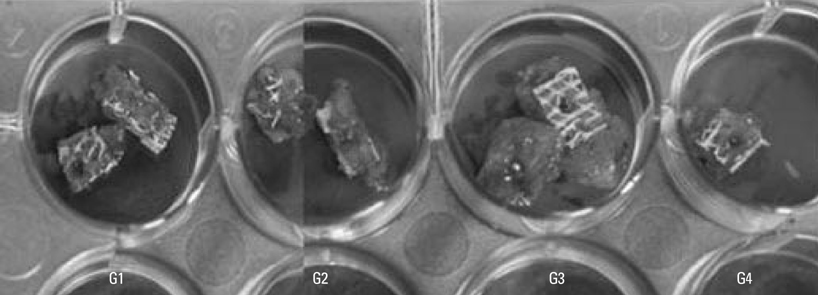
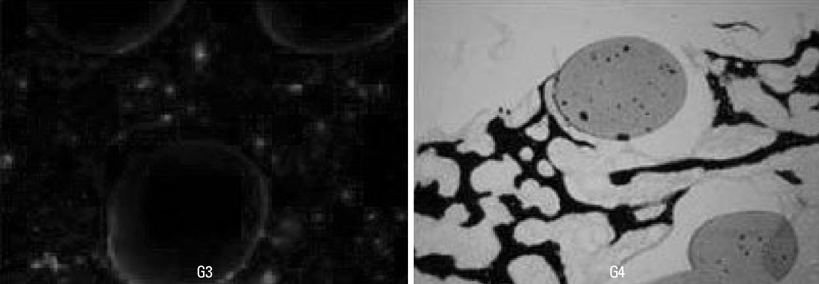
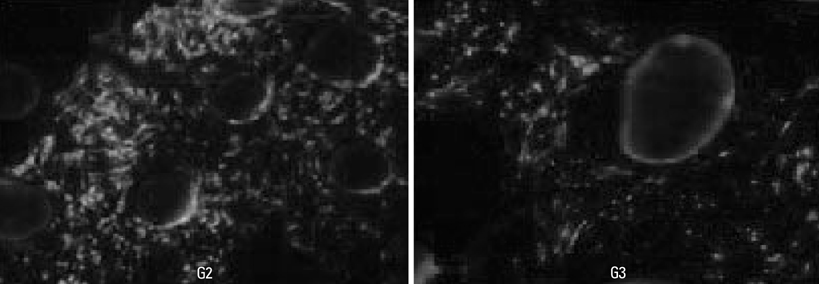
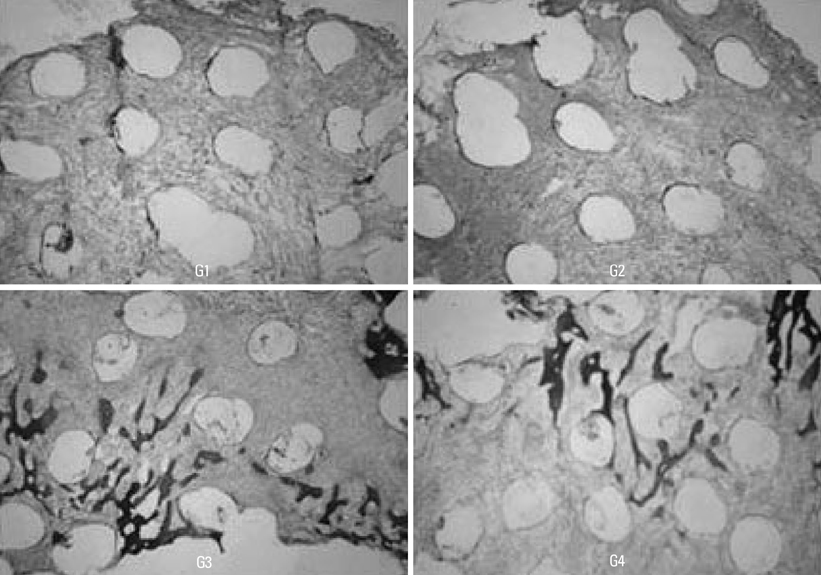
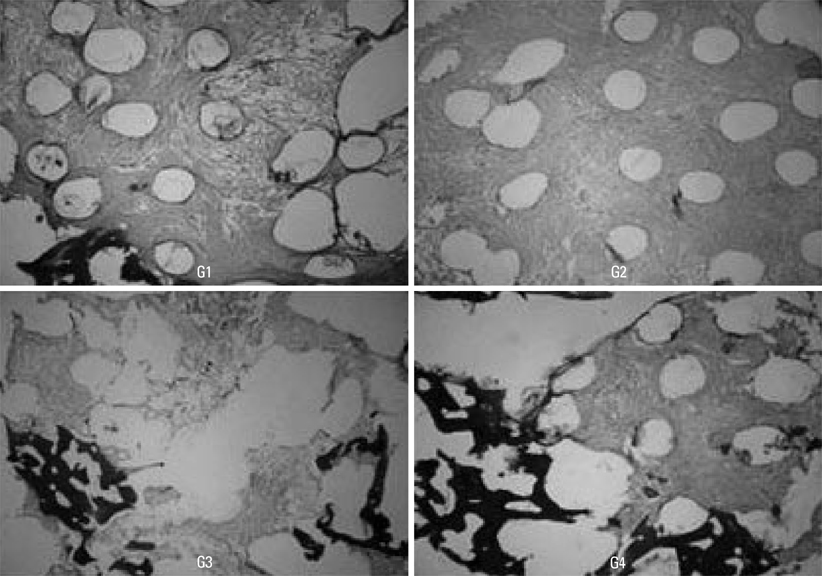
The subjects were two beagle dogs. Total dose of rhBMP-2 on each block was 10 microg with 50 microg/mg concentration. The cortical bone of the scapula of the dog was removed which was the same size of PCL-TCP block (Osteopore International Pte, Singapore; 5.0x5.0x8.0 mm in size), and the following graft material then was fixed with orthodontic mini-implant, Dual-top(R) (Titanium alloy, Jeil Co. Seoul, Korea). Four experimental groups were prepared for this study, Group 1: PCL-TCP + aFG; Group 2: PCL-TCP + aFG + dMSCs; Group 3: PCL-TCP + aFG + dMSCs + rhBMP-2; Group 4: PCL-TCP + aFG + dMSCs + rhBMP-2 + PCL membrane. The survival or proliferation of dMSCs cells was identified with an extracted tissue through a fluorescence microscope, H-E staining and Von-Kossa staining in two weeks and four weeks after the transplantation.
RESULTS
The survival and proliferation of dMSCs were identified through a fluorescence microscope from both Group 1 and Group 2 in two weeks and four weeks after the transplantation. Histological observation also found that the injected cells were proliferating well in the G2, G3, and G4 scaffolds.
CONCLUSION
This study concluded that bone ingrowth occurred in PCL-TCP scaffold which was transplanted with rhBMP-2, and MSCs did not affect bone growth. More sufficient healing time would be needed to recognize effects of dMSCs on bone formation.
Keyword
MeSH Terms
-
Animals
Bone Morphogenetic Proteins/*pharmacology
Calcium Phosphates/*pharmacology
Cell Proliferation/drug effects
Cell Survival/drug effects
Cells, Cultured
Dogs
Fibrin Tissue Adhesive/pharmacology
Humans
Mesenchymal Stem Cell Transplantation
Mesenchymal Stem Cells/*cytology/*drug effects/physiology
Microscopy, Fluorescence
Osteogenesis/*drug effects
Polyesters/*pharmacology
Recombinant Proteins/*pharmacology
Transforming Growth Factor beta/*pharmacology
Figure
Cited by 3 articles
-
Regenerative medicine for the reconstruction of hard tissue defects in oral and maxillofacial surgery
Young-Kyun Kim
J Korean Assoc Oral Maxillofac Surg. 2012;38(2):69-70. doi: 10.5125/jkaoms.2012.38.2.69.Assessment of stem cell viability in the initial healing period in rabbits with a cranial bone defect according to the type and form of scaffold
Seung-Hwan Kang, Jun-Beom Park, InSoo Kim, Won Lee, Heesung Kim
J Periodontal Implant Sci. 2019;49(4):258-267. doi: 10.5051/jpis.2019.49.4.258.Tooth-derived bone graft material
Young-Kyun Kim, Junho Lee, In-Woong Um, Kyung-Wook Kim, Masaru Murata, Toshiyuki Akazawa, Masaharu Mitsugi
J Korean Assoc Oral Maxillofac Surg. 2013;39(3):103-111. doi: 10.5125/jkaoms.2013.39.3.103.
Reference
-
1. Jeong SI, Kim BS, Kang SW, Kwon JH, Lee YM, Kim SH, et al. In vivo biocompatibility and degradation behavior of elastic poly (L-lactide-co-epsilon-caprolactone) scaffolds. Biomaterials. 2004. 25:5939–5946.
Article2. Gabriel M, van Nieuw Amerongen GP, Van Hinsbergh VW, Amerongen AV, Zentner A. Direct grafting of RGD-motif-containing peptide on the surface of polycaprolactone films. J Biomater Sci Polym Ed. 2006. 17:567–577.
Article3. Rai B, Teoh SH, Ho KH, Hutmacher DW, Cao T, Chen F, et al. The effect of rhBMP-2 on canine osteoblasts seeded onto 3D bioactive polycaprolactone scaffolds. Biomaterials. 2004. 25:5499–5506.
Article4. Lee SJ. Cytokine delivery and tissue engineering. Yonsei Med J. 2000. 41:704–719.
Article5. Pang EK, Im SU, Kim CS, Choi SH, Chai JK, Kim CK, et al. Effect of recombinant human bone morphogenetic protein-4 dose of bone formation in a rat calvarial defect model. J Periodontol. 2004. 75:1364–1370.
Article6. Orban JM, Marra KG, Hollinger JO. Composition options for tissue-engineered bone. Tissue Eng. 2002. 8:529–539.
Article7. Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991. 9:641–650.
Article8. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.
Article9. Kim CS, Kim JI, Kim J, Choi SH, Chai JK, Kim CK, et al. Ectopic bone formation associated with recombinant human bone morphogenetic proteins-2 using absorbable collagen sponge and beta tricalcium phosphate as carriers. Biomaterials. 2005. 26:2501–2507.
Article10. Barboza EP, Duarte ME, Geolás L, Sorensen RG, Riedel GE, Wikesjö UM. Ridge augmentation following implantation of recombinant human bone morphogenetic protein-2 in the dog. J Periodontol. 2000. 71:488–496.
Article11. Petite H, Viateau V, Bensaïd W, Meunier A, de Pollak C, Bourguignon M, et al. Tissue-engineered bone regeneration. Nat Biotechnol. 2000. 18:959–963.12. Yeo A, Sju E, Rai B, Teoh SH. Customizing the degradation and load-bearing profile of 3D polycaprolactone-tricalcium phosphate scaffolds under enzymatic and hydrolytic conditions. J Biomed Mater Res B Appl Biomater. 2008. 87:562–569.13. Rai B, Teoh SH, Hutmacher DW, Cao T, Ho KH. Novel PCL-based honeycomb scaffolds as drug delivery systems for rhBMP-2. Biomaterials. 2005. 26:3739–3748.
Article14. Rai B, Teoh SH, Ho KH. An in vitro evaluation of PCL-TCP composites as delivery systems for platelet-rich plasma. J Control Release. 2005. 107:330–342.
Article15. Chiapasco M, Abati S, Romeo E, Vogel G. Clinical outcome of autogenous bone blocks or guided bone regeneration with e-PTFE membranes for the reconstruction of narrow edentulous ridges. Clin Oral Implants Res. 1999. 10:278–288.
Article16. Rah DK. Art of replacing craniofacial bone defects. Yonsei Med J. 2000. 41:756–765.
Article17. Song L, Baksh D, Tuan RS. Mesenchymal stem cell-based cartilage tissue engineering: cells, scaffold and biology. Cytotherapy. 2004. 6:596–601.
Article18. Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998. 9:49–61.
Article19. Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004. 22:233–241.
Article20. Deckers MM, van Bezooijen RL, van der Horst G, Hoogendam J, van Der Bent C, Papapoulos SE, et al. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology. 2002. 143:1545–1553.
Article21. Bösch P, Lintner F, Arbes H, Brand G. Experimental investigations of the effect of the fibrin adhesive on the Kiel heterologous bone graft. Arch Orthop Trauma Surg. 1980. 96:177–185.
Article22. Isogai N, Landis WJ, Mori R, Gotoh Y, Gerstenfeld LC, Upton J, et al. Experimental use of fibrin glue to induce site-directed osteogenesis from cultured periosteal cells. Plast Reconstr Surg. 2000. 105:953–963.
Article23. Reiss RF, Oz MC. Autologous fibrin glue: production and clinical use. Transfus Med Rev. 1996. 10:85–92.
Article24. Yoshida H, Kamiya A. A quicker preparation method for autologous fibrin glue. Biol Pharm Bull. 1998. 21:1367–1370.
Article25. Thorn JJ, Sørensen H, Weis-Fogh U, Andersen M. Autologous fibrin glue with growth factors in reconstructive maxillofacial surgery. Int J Oral Maxillofac Surg. 2004. 33:95–100.
Article26. Orban JM, Marra KG, Hollinger JO. Composition options for tissue-engineered bone. Tissue Eng. 2002. 8:529–539.
Article27. Patel VV, Zhao L, Wong P, Kanim L, Bae HW, Pradhan BB, et al. Controlling bone morphogenetic protein diffusion and bone morphogenetic protein-stimulated bone growth using fibrin glue. Spine (Phila Pa 1976). 2006. 31:1201–1206.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bone Healing in Ovariectomized-rabbit Calvarial Defect with Tricalcium Phosphate Coated with Recombinant Human Bone Morphogenetic Protein-2 Genetically Engineered in Escherichia coli
- Use of stem-cell sheets expressing bone morphogenetic protein-7 in the management of a nonunion radial fracture in a Toy Poodle
- Periimplant Bone Regeneration in Hydroxyapatite Block Grafts with Mesenchymal Stem Cells and Bone Morphogenetic Protein-2
- Spinal Fusion Based on Ex Vivo Gene Therapy Using Recombinant Human BMP Adenoviruses
- Development of Refolding Process to Obtain Active Recombinant Human Bone Morphogenetic Protein-2 and its Osteogenic Efficacy on Oral Stem Cells