Inflammatory Bowel Diseases and Enteric Microbiota
- Affiliations
-
- 1Department of Microbiology, Hanyang University College of Medicine, Seoul, Korea. jungmogg@hanyang.ac.kr
- KMID: 1775921
- DOI: http://doi.org/10.4166/kjg.2010.55.1.4
Abstract
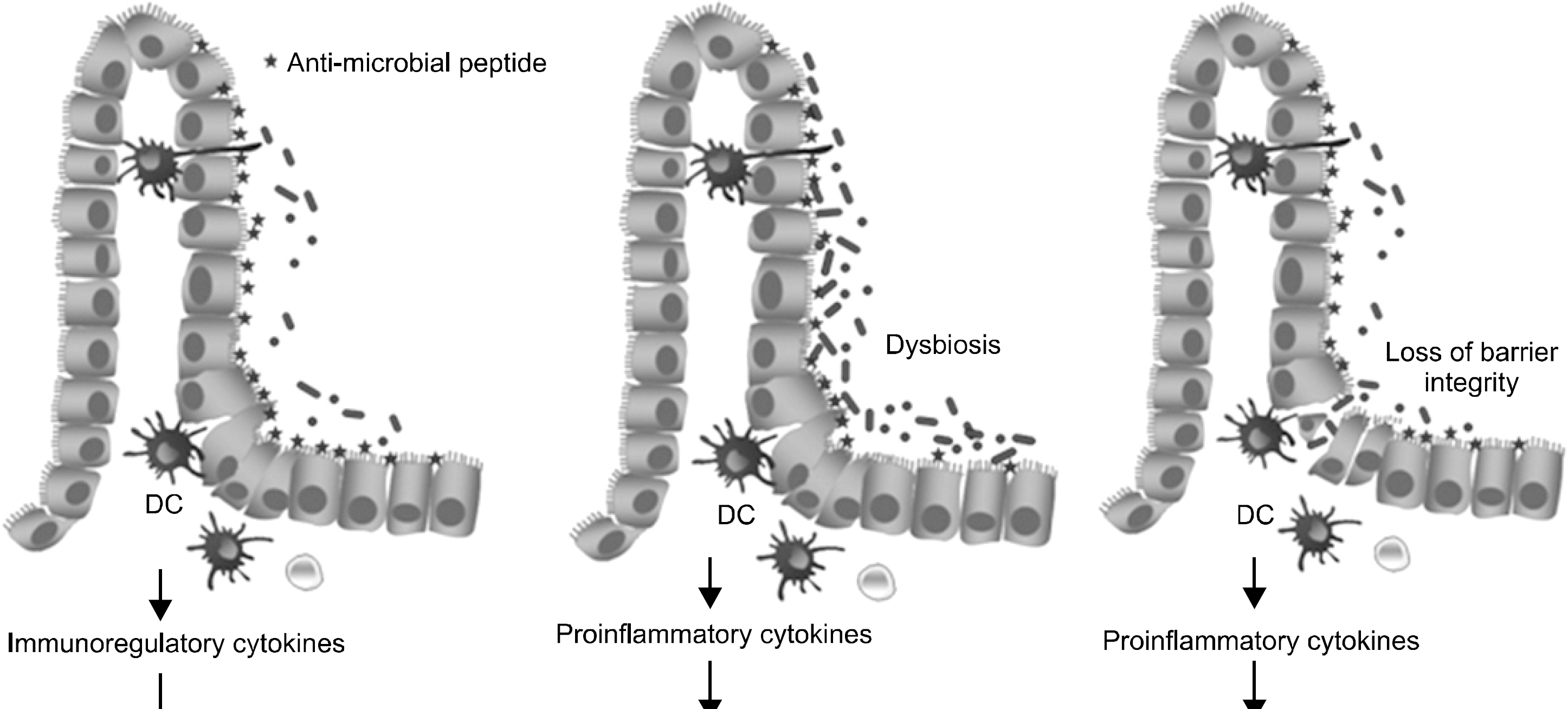
- Intestinal mucosal layers are colonized by a complex microbiota that provides beneficial effects under normal physiological conditions, but is capable of contributing to chronic inflammatory disease such as inflammatory bowel disease (IBD) in susceptible individuals. Studies have shown that the enteric microbiota may drive the development of the gut immune system and can induce immune homeostasis as well as contribute to the development of IBD although the precise etiology is still unknown. Therefore, intestinal microbes seem to play a key role in the disease pathogenesis. Especially, dysbiosis, which is a shift in the composition of enteric microbiota to a nonphysiologic composition, is associated with one or more defects in mucosal immune functions, including microbe recognition, barrier function, intercellular communication, and anti-microbial effector mechanisms. This review focuses on the impact of enteric microbiota on the development and perpetuation of IBD. In addition, interactions with enteric bacteria and mucosal cells, including intestinal epithelial cells, dendritic cells, and T cells, to induce immune responses at mucosal surfaces have been discussed in the point of IBD pathogenesis. Further extension of the knowledge of enteric microbiota may lead to insights on the pathogenesis and new therapeutic strategies for IBD.
MeSH Terms
Figure
Cited by 5 articles
-
FOXP3+T Cells and TGF-β1 in Colonic Mucosa of Children with Crohn's Disease
Joo Hyun Gil, Jung Eun Oh, Jeong Wan Seo, Min-Sun Cho, Ky Young Cho, Eun Sun Yoo
Korean J Pediatr Gastroenterol Nutr. 2011;14(3):258-268. doi: 10.5223/kjpgn.2011.14.3.258.Inflammatory Bowel Diseases and Inflammasome
Jung Mogg Kim
Korean J Gastroenterol. 2011;58(6):300-310. doi: 10.4166/kjg.2011.58.6.300.Roles of Enteric Microbial Composition and Metabolism in Health and Diseases
Jung Mogg Kim
Korean J Gastroenterol. 2013;62(4):191-205. doi: 10.4166/kjg.2013.62.4.191.(Xylan-regulated Delivery of Human Keratinocyte Growth Factor-2 to the Inflamed Colon by the Human Anaerobic Commensal Bacterium
Bacteroides ovatus . Gut 2010;59:461-469)
Korean J Gastroenterol. 2010;56(6):394-396. doi: 10.4166/kjg.2010.56.6.394.Antimicrobial Proteins in Intestine and Inflammatory Bowel Diseases
Jung Mogg Kim
Intest Res. 2014;12(1):20-33. doi: 10.5217/ir.2014.12.1.20.
Reference
-
1. Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008; 134:577–594.
Article2. Manichanh C, Rigottier-Gois L, Bonnaud E, et al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut. 2006; 55:205–211.
Article3. Kleessen B, Kroesen AJ, Buhr HJ, Blaut M. Mucosal and invading bacteria in patients with inflammatory bowel disease compared with controls. Scand J Gastroenterol. 2002; 37:1034–1041.
Article4. Pène J, Chevalier S, Preisser L, et al. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J Immunol. 2008; 180:7423–7430.
Article5. Kobayashi T, Okamoto S, Hisamatsu T, et al. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn's disease. Gut. 2008. 1682–1689.
Article6. Shale M, Ghosh S. Beyond TNF, Th1 and Th2 in inflammatory bowel disease. Gut. 2008. 1349–1351.
Article7. Rutgeerts P, Goboes K, Peeters M, et al. Effect of faecal stream diversion on recurrence of Crohn's disease in the ne-oterminal ileum. Lancet. 1991; 28(338):771–774.
Article8. D'Haens GR, Geboes K, Peeters M, et al. Early lesions of recurrent Crohn's disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology. 1998; 114:262–267.9. Salzman NH, Bevins CL. Negative interactions with the microbiota: IBD. Adv Exp Med Biol. 2008; 635:67–78.
Article10. Sears CL. A dynamic partnership: celebrating our gut flora. Anaerobe. 2005; 11:247–251.
Article11. Ott SJ, Musfeldt M, Wenderoth DF, et al. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004; 53:685–693.
Article12. Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005; 308:1635–1638.
Article13. Duncan SH, Louis P, Flint HJ. Cultivable bacterial diversity from the human colon. Lett Appl Microbiol. 2007; 44:343–350.
Article14. Kurokawa K, Itoh T, Kuwahara T, et al. Comparative meta-genomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res. 2007; 14:169–181.
Article15. Frank DN, St Amand AL, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007; 104:13780–13785.
Article16. Tamboli CP, Neut C, Desreumaux P, Colombel JF. Dysbiosis as a prerequisite for IBD. Gut. 2004; 53:1057.17. Scanlan PD, Marchesi JR. Micro-eukaryotic diversity of the human distal gut microbiota: qualitative assessment using culture-dependent and -independent analysis of faeces. ISME J. 2008; 2:1183–1193.
Article18. Franke A, Balschun T, Karlsen TH, et al. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet. 2008; 40:1319–1323.
Article19. Frank DN, St Amand AL, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007; 104:13780–13785.
Article20. Kühbacher T, Ott SJ, Helwig U, et al. Bacterial and fungal microbiota in relation to probiotic therapy (VSL#3) in pouchitis. Gut. 2006; 55:833–841.
Article21. Ott SJ, Kühbacher T, Musfeldt M, et al. Fungi and inflammatory bowel diseases: alterations of composition and diversity. Scand J Gastroenterol. 2008; 43:831–841.
Article22. Ott SJ, Musfeldt M, Wenderoth DF, et al. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004; 53:685–693.
Article23. Rath HC, Schultz M, Freitag R, et al. Different subsets of enteric bacteria induce and perpetuate experimental colitis in rats and mice. Infect Immun. 2001; 69:2277–2285.
Article24. Scanlan PD, Shanahan F, O'Mahony C, Marchesi JR. Culture-independent analyses of temporal variation of the dominant fecal microbiota and targeted bacterial subgroups in Crohn's disease. J Clin Microbiol. 2006; 44:3980–3988.
Article25. Balish E, Warner T. Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. Am J Pathol. 2002; 160:2253–2257.26. Kim SC, Tonkonogy SL, Albright CA, et al. Variable phenotypes of enterocolitis in interleukin 10-deficient mice monoassociated with two different commensal bacteria. Gastroenterology. 2005; 128:891–906.
Article27. Burich A, Hershberg R, Waggie K, et al. Helicobacter-induced inflammatory bowel disease in IL-10- and T cell-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2001; 281:764–778.28. Prindiville T, Cantrell M, Wilson KH. Ribosomal DNA sequence analysis of mucosa-associated bacteria in Crohn's disease. Inflamm Bowel Dis. 2004; 10:824–833.
Article29. Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol. 2005; 43:3380–3389.
Article30. Seksik P, Lepage P, de la Cochetière MF. Search for localized dysbiosis in Crohn's disease ulcerations by temporal temperature gradient gel electrophoresis of 16S rRNA. J Clin Microbiol. 2005; 43:4654–4658.
Article31. Gophna U, Sommerfeld K, Gophna S, et al. Differences between tissue-associated intestinal microfloras of patients with Crohn's disease and ulcerative colitis. J Clin Microbiol. 2006; 44:4136–4141.
Article32. Bibiloni R, Mangold M, Madsen KL, Fedorak RN, Tannock GW. The bacteriology of biopsies differs between newly diagnosed, untreated, Crohn's disease and ulcerative colitis patients. J Med Microbiol. 2006; 55:1141–1149.
Article33. Kotlowski R, Bernstein CN, Sepehri S, Krause DO. High prevalence of Escherichia coli belonging to the B2+D phylogenetic group in inflammatory bowel disease. Gut. 2007; 56:669–675.34. Martin HM, Campbell BJ, Hart CA, et al. Enhanced Escherichia coli adherence and invasion in Crohn's disease and colon cancer. Gastroenterology. 2004; 127:80–93.35. Darfeuille-Michaud A, Boudeau J, Bulois P, et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology. 2004; 127:412–421.36. Conte MP, Schippa S, Zamboni I, et al. Gut-associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut. 2006; 55:1760–1767.
Article37. Mylonaki M, Rayment NB, Rampton DS, Hudspith BN, Brostoff J. Molecular characterization of rectal mucosa-associated bacterial flora in inflammatory bowel disease. Inflamm Bowel Dis. 2005; 11:481–487.
Article38. Burke DA, Axon AT. Adhesive Escherichia coli in inflammatory bowel disease and infective diarrhoea. BMJ. 1988; 297:102–104.
Article39. Giaffer MH, Holdsworth CD, Duerden BI. Virulence properties of Escherichia coli strains isolated from patients with inflammatory bowel disease. Gut. 1992; 33:646–650.40. Hartley MG, Hudson MJ, Swarbrick ET. Adhesive and hydrophobic properties of Escherichia coli from the rectal mucosa of patients with ulcerative colitis. Gut. 1993; 34:63–67.41. Schultsz C, Moussa M, van Ketel R, Tytgat GN, Dankert J. Frequency of pathogenic and enteroadherent Escherichia coli in patients with inflammatory bowel disease and controls. J Clin Pathol. 1997; 50:573–579.42. Walmsley RS, Anthony A, Sim R, Pounder RE, Wakefield AJ. Absence of Escherichia coli, Listeria monocytogenes, and Klebsiella pneumoniae antigens within inflammatory bowel disease tissues. J Clin Pathol. 1998; 51:657–661.43. Subramanian S, Campbell BJ, Rhodes JM. Bacteria in the pathogenesis of inflammatory bowel disease. Curr Opin Infect Dis. 2006; 19:475–484.
Article44. Darfeuille-Michaud A, Neut C, Barnich N, et al. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology. 1998; 115:1405–1413.45. Cohavy O, Bruckner D, Gordon LK, et al. Colonic bacteria express an ulcerative colitis pANCA-related protein epitope. Infect Immun. 2000; 68:1542–1548.
Article46. Abad E, Tural C, Mirapeix E, Cuxart A. Relationship between ANCA and clinical activity in inflammatory bowel disease: variation in prevalence of ANCA and evidence of heterogeneity. J Autoimmun. 1997; 10:175–180.
Article47. Rolhion N, Darfeuille-Michaud A. Adherent-invasive Escherichia coli in inflammatory bowel disease. Inflamm Bowel Dis. 2007; 13:1277–1283.48. Glasser AL, Boudeau J, Barnich N, et al. Adherent invasive Escherichia coli strains from patients with Crohn's disease survive and replicate within macrophages without inducing host cell death. Infect Immun. 2001; 69:5529–5537.49. Belsheim MR, Darwish RZ, Watson WC, Schieven B. Bacterial L-form isolation from inflammatory bowel disease patients. Gastroenterology. 1983; 85:364–369.
Article50. Sasaki M, Sitaraman SV, Babbin BA, et al. Invasive Escherichia coli are a feature of Crohn's disease. Lab Invest. 2007; 87:1042–1054.51. Subramanian S, Rhodes JM, Hart CA, et al. Characterization of epithelial IL-8 response to inflammatory bowel disease mucosal E. coli and its inhibition by mesalamine. Inflamm Bowel Dis. 2008; 14:162–175.52. Lodes MJ, Cong Y, Elson CO, et al. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004; 113:1296–1306.
Article53. Eaves-Pyles T, Allen CA, Taormina J, et al. Escherichia coli isolated from a Crohn's disease patient adheres, invades, and induces inflammatory responses in polarized intestinal epithelial cells. Int J Med Microbiol. 2008; 298:397–409.54. Dalziel TK. Chronic intestinal enteritis. Br Med J. 1913; 2:1068–1070.55. Greenstein RJ. Is Crohn's disease caused by a mycobacterium? Comparisons with leprosy, tuberculosis and Johne's disease. Lancet Infect Dis. 2003; 3:507–514.
Article56. Scanu AM, Bull TJ, Cannas S, Sanderson JD, et al. Myco-bacterium avium subspecies paratuberculosis infection in irritable bowel syndrome and comparison with Crohn's and Johne's diseases: common neural and immune pathogenicities. J Clin Microbiol. 2007; 45:3883–3890.57. Naser SA, Ghobrial G, Romero C, Valentine JF. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn's disease. Lancet. 2004; 364:1039–1044.58. Chiodini RJ, Van Kruiningen HJ, Thayer WR, Coutu JA. Spheroplastic phase of mycobacteria isolated from patients with Crohn's disease. J Clin Microbiol. 1986; 24:357–363.
Article59. Sechi LA, Scanu AM, Molicotti P, et al. Detection and Isolation of Mycobacterium avium subspecies paratuberculosis from intestinal mucosal biopsies of patients with and without Crohn's disease in Sardinia. Am J Gastroenterol. 2005; 100:1529–1536.60. Elsaghier A, Prantera C, Moreno C, Ivanyi J. Antibodies to Mycobacterium paratuberculosis-specific protein antigens in Crohn's disease. Clin Exp Immunol. 1992; 90:503–508.61. Schwartz D, Shafran I, Romero C, et al. Use of short-term culture for identification of Mycobacterium avium subsp. paratuberculosis in tissue from Crohn's disease patients. Clin Microbiol Infect. 2000; 6:303–307.62. Naser S, Shafran I, El-Zaatari F. Mycobacterium avium subsp. paratuberculosis in Crohn's disease is serologically positive. Clin Diagn Lab Immunol. 1999; 6:282.63. Abubakar I, Myhill D, Aliyu SH, Hunter PR. Detection of Mycobacterium avium subspecies paratuberculosis from patients with Crohn's disease using nucleic acid-based techniques: a systematic review and metaanalysis. Inflamm Bowel Dis. 2008; 14:401–410.64. Mendoza JL, Lana R, Díaz-Rubio M. Mycobacterium avium subspecies paratuberculosis and its relationship with Crohn's disease. World J Gastroenterol. 2009; 15:417–422.65. Hulten K, El-Zimaity HM, Karttunen TJ, et al. Detection of Mycobacterium avium subsp. paratuberculosis in Crohn's diseased tissues by in situ hybridization. Am J Gastroenterol. 2001; 96:1529–1535.66. Dell'Isola B, Poyart C, Goulet O, et al. Detection of Mycobacterium paratuberculosis by polymerase chain reaction in children with Crohn's disease. J Infect Dis. 1994; 169:449–451.67. Lisby G, Andersen J, Engbaek K, Binder V. Mycobacterium paratuberculosis in intestinal tissue from patients with Crohn's disease demonstrated by a nested primer polymerase chain reaction. Scand J Gastroenterol. 1994; 29:923–929.68. Macfarlane S, Steed H, Macfarlane GT. Intestinal bacteria and inflammatory bowel disease. Crit Rev Clin Lab Sci. 2009; 46:25–54.
Article69. Markesich DC, Graham DY, Yoshimura HH. Progress in culture and subculture of spheroplasts and fastidious acid fast bacilli isolated from intestinal tissues. J Clin Microbiol. 1988; 26:1600–1603.70. Gitnick G, Collins J, Beaman B, et al. Preliminary report on isolation of mycobacteria from patients with Crohn's disease. Dig Dis Sci. 1989; 34:925–932.
Article71. Green EP, Tizard MLV, Moss MT, et al. Sequence and characteristics of IS900, an insertion identified in a human Crohn's disease isolate of Mycobacterium paratuberculosis. Nucleic Acid Res. 1989; 17:9063–9073.72. Kunze ZM, Wall S, Appelberg R, et al. IS901, a new member of a widespread class of atypical insertion sequences is associated with pathogenicity in Mycobacterium avium. Mol Microbiol. 1991; 5:2265–2272.73. Hermon-Taylor J, Bull TJ, Sheridan JM, et al. Causation of Crohn's disease by Mycobacterium avium subspecies paratuberculosis. Can J Gastroenterol. 2000; 14:521–539.74. Jeyanathan M, Alexander DC, Turenne CY, Girard C, Behr MA. Evaluation of in situ methods used to detect Mycobacterium avium subsp. paratuberculosis in samples from patients with Crohn's Disease. J Clin Microbiol. 2006; 44:2942–2950.75. Sanderson JD, Moss MT, Tizard ML, Hermon-Taylor J. Mycobacterium paratuberculosis DNA in Crohn's disease tissue. Gut. 1992; 33:890–896.76. Cousins DV, Whittington R, Marsh I, et al. Mycobacteria distinct from Mycobacterium avium subsp. paratuberculosis isolated from the faeces of ruminants possess IS900-like sequences detectable by IS900 polymerase chain reaction: implications for diagnosis. Mol Cell Probes. 1999; 13:431–442.77. Englund S, Bolske G, Johansson KE. An IS900-like sequence found in a Mycobacterium sp. other than Mycobacterium avium subsp. paratuberculosis. FEMS Microbiol Lett. 2002; 209:267–271.78. Peeters M, Joossens S, Vermeire S, et al. Diagnostic value of anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease. Am J Gastroenterol. 2001; 96:730–734.79. Bernstein CN, Blanchard JF, Rawsthorne P, Collins MT. Population-based case control study of seroprevalence of Mycobacterium paratuberculosis in patients with Crohn's disease and ulcerative colitis. J Clin Microbial. 2004; 42:1129–1135.80. Clancy R, Ren Z, Turton J, Pang G, Wettstein A. Molecular evidence for Mycobacterium avium subspecies paratuberculosis (MAP) in Crohn's disease correlates with enhanced TNF-a secretion. Dig Liver Dis. 2007; 39:445–451.81. Ogura Y, Bonen DK, Inohara N, et al. A frame shift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001; 411:603–606.82. Sechi LA, Gazouli M, Ikonomopolous JC, et al. Mycobacterium avium subsp. paratuberculosis, genetic susceptibility to Crohn's disease, and Sardinians: the way ahead. J Clin Microbiol. 2005; 43:5275–5277.83. Ferwerda G, Kullberg BJ, de Jong DJ, et al. Mycobacterium paratuberculosis is recognized by Toll-like receptors and NOD2. J Leucocyte Biol. 2007; 82:1011–1018.84. Mpofu CM, Campbell BJ, Subramanian S, et al. Microbial mannan inhibits bacterial killing by macrophages: a possible pathogenic mechanism for Crohn's disease. Gastroenterology. 2007; 133:1487–1498.
Article85. Marcus R, Watt J. Seaweeds and ulcerative colitis in laboratory animals. Lancet. 1969; 2:489–490.
Article86. Marcus AJ, Marcus SN, Marcus R, Watt J. Rapid production of ulcerative disease of the colon in newly-weaned guinea-pigs by degraded carrageenan. J Pharm Pharmacol. 1989; 41:42342–42346.
Article87. Watt J, Marcus R. Experimental ulcerative disease of the colon in animals. Gut. 1973; 14:506–510.
Article88. Gebhart CJ, Barns SM, McOrist S, Lin GF, Lawson GHK. Ileal Symbiont Intracellularis, an obligate intracellular bacterium of porcine intestines showing a relationship to Desulfovibrio species. Int J Syst Bacteriol. 1993; 43:533–538.89. Fox JG, Dewhirst FE, Fraser GJ, et al. Intracellular Campylobacter -like organism from ferrets and hamsters with proliferative bowel disease is a Desulfovibrio spp. J Clin Microbiol. 1994; 32:1229–1237.90. Pitcher MCL, Beatty ER, Cummings JH. The contribution of sulphate reducing bacteria and 5-aminosalicylic acid to faecal sulphide in patients with ulcerative colitis. Gut. 2000; 46:64–72.
Article91. Loubinoux J, Bronowicji JP, Pereira IAC, Moungenel JL, Faou AE. Sulfate-reducing bacteria in human feces and their association with inflammatory diseases. FEMS Microbiol Ecol. 2002; 40:107–112.92. Pitcher MCL, Beatty ER, Harris RM, Waring RH, Cummings JH. Sulfur metabolism in ulcerative colitis. Investigation of detoxification enzymes in peripheral blood. Dig Dis Sci. 1998; 43:2080–2085.93. Levitt MD, Furne J, Springfield J, Suarez F, DeMaster E. Detoxification of hydrogen sulfide and methanethiol in the cecal mucosa. J Clin Invest. 1999; 104:1107–1114.
Article94. Gardiner KR, Halliday MI, Barclay GR, et al. Significance of systemic endotoxaemia in inflammatory bowel disease. Gut. 1996; 36:897–901.
Article95. Christl S, Eisner HD, Kasper H, Scheppach W. Antagonistic effects of sulfide and butyrate on proliferation of colonic mucosa: a potential role for these agents in the pathogenesis of ulcerative colitis. Dig Dis Sci. 1996; 41:2477–2481.96. Dzierzewicz Z, Cwalina B, Weglarz L, Wisniowska B, Szczerba J. Susceptibility of Desulfovibrio desulfuricans intestinal strains to sulfasalazine and its biotransformation products. Med Sci Mon. 2004; 10:185–190.97. Oghe H, Furne J, Springfield J, et al. Association between fecal hydrogen sulfide production and pouchitis. Dis Col Rect. 2005; 48:469–475.98. Gibson GR, Cummings JH, Macfarlane GT. Growth and activities of sulphate reducing bacteria in gut contents from healthy subjects and patients with ulcerative colitis. FEMS Microbiol Ecol. 1991; 86:103–112.99. McKenzie H, Main J, Pennington CR, Parratt D. Antibody to selected strains of Saccharomyces cerevisiae (baker's and brewer's yeast) and Candida albicans in Crohn's disease. Gut. 1990; 31:536–538.100. Adams RJ, Heazlewood SP, Gilshenan KS, et al. IgG antibodies against common gut bacteria are more diagnostic for Crohn's disease than IgG against mannan or flagellin. Am J Gastroenterol. 2008; 103:386–396.
Article101. Sears CL. Enterotoxigenic Bacteroides fragilis: a rogue among symbiotes. Clin Microbiol Rev. 2009; 22:349–369.102. Prindiville TP, Sheikh RA, Cohen SH, et al. Bacteroides fragilis enterotoxin gene sequences in patients with inflammatory bowel disease. Emerg Infect Dis. 2000; 6:171–174.103. Basset C, Holton J, Bazeos A, et al. Are Helicobacter species and enterotoxigenic Bacteroides fragilis involved in inflammatory bowel disease? Dig Dis Sci. 2004; 49:1425–1432.104. Rabizadeh S, Rhee KJ, Wu S, et al. Enterotoxigenic Bacteroides fragilis: a potential instigator of colitis. Inflamm Bowel Dis. 2007; 13:1475–1483.105. Kim JM, Oh YK, Kim YJ, Oh HB, Cho YJ. Polarized secretion of CXC chemokines by human intestinal epithelial cells in response to Bacteroides fragilis enterotoxin: NF-κB plays a major role in the regulation of IL-8 expression. Clin Exp Immunol. 2001; 123:421–427.106. Kim JM, Cho SJ, Oh YK, et al. Nuclear factor-κB activation pathway in intestinal epithelial cells is a major regulator of chemokine gene expression and neutrophil migration induced by Bacteroides fragilis enterotoxin. Clin Exp Immunol. 2002; 130:59–66.107. Kim JM, Jung HY, Lee JY, et al. Mitogen-activated protein kinase and activator protein-1 dependent signals are essential for Bacteroides fragilis enterotoxin-induced enteritis. Eur J Immunol. 2005; 35:2648–2657.108. Kim JM, Lee JY, Yoon YM, et al. Bacteroides fragilis enterotoxin induces cyclooxygenase-2 and fluid secretion in intestinal epithelial cells through NF-kappaB activation. Eur J Immunol. 2006; 36:2446–2456.109. Kim JM, Lee DH, Kim JS, et al. 5,7-dihydroxy-3,4,6-tri-methoxyflavone inhibits the inflammatory effects induced by Bacteroides fragilis enterotoxin via dissociating the complex of heat shock protein 90 and IκBα and IκB kinase-gamma in intestinal epithelial cell culture. Clin Exp Immunol. 2009; 155:541–551.110. Wu S, Powell J, Mathioudakis N, et al. Bacteroides fragilis enterotoxin induces intestinal epithelial cell secretion of interleukin-8 through mitogen-activated protein kinases and a tyrosine kinase-regulated nuclear factor-κB pathway. Infect Immun. 2004; 72:5832–5839.111. Kim JM, Lee JY, Kim YJ. Inhibition of apoptosis in Bacteroides fragilis enterotoxin-stimulated intestinal epithelial cells through the induction of c-IAP-2. Eur J Immunol. 2008; 38:2190–2199.112. Wu S, Rhee KJ, Albesiano E, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009; 15:1016–1022.
Article113. Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008; 8:411–420.
Article114. Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006; 3:390–407.
Article115. Clavel T, Haller D. Bacteria- and host-derived mechanisms to control intestinal epithelial cell homeostasis: implications for chronic inflammation. Inflamm Bowel Dis. 2007; 13:1153–1164.
Article116. Cario E. Bacterial interactions with cells of the intestinal mucosa: Toll-like receptors and NOD2. Gut. 2005; 54:11821193.
Article117. Cario E, Podolsky DK. Intestinal epithelial TOLLerance versus inTOLLerance of commensals. Mol Immunol. 2005; 42:887–893.
Article118. Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006; 313:1126–1130.
Article119. Gironella M, Iovanna JL, Sans M, Gil F, et al. Anti-inflammatory effects of pancreatitis associated protein in inflammatory bowel disease. Gut. 2005; 54:1244–1253.
Article120. Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003; 197:1107–1117.
Article121. Underhill D, Braun J. Current understanding of fungal microflora in inflammatory bowel disease pathogenesis. Inflamm Bowel Dis. 2008; 14:1147–1153.
Article122. Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006 24; 124:837–848.
Article123. Magalhaes JG, Tattoli I, Girardin SE. The intestinal epithelial barrier: how to distinguish between the microbial flora and pathogens. Semin Immunol. 2007; 19:106–115.
Article124. Kelly D, Campbell JI, King TP, et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol. 2004; 5:104–112.125. Kelly D, Conway S, Aminov R. Commensal gut bacteria: mechanisms of immune modulation. Trends Immunol. 2005; 26:326–333.
Article126. Kumar A, Wu H, Collier-Hyams LS, et al. Commensal bacteria modulate cullindependent signaling via generation of reactive oxygen species. EMBO J. 2007; 26:4457–4466.
Article127. Otte JM, Cario E, Podolsky DK. Mechanisms of cross hypo-responsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology. 2004; 126:1054–1570.
Article128. Stoll M, Corneliussen B, Costello CM, et al. Genetic variation in DLG5 is associated with inflammatory bowel disease. Nat Genet. 2004; 36:476–480.
Article129. Peltekova VD, Wintle RF, Rubin LA, et al. Functional variants of OCTN cation transporter genes are associated with Crohn disease. Nat Genet. 2004; 36:471–475.
Article130. Russell RK, Drummond HE, Nimmo ER, et al. Analysis of the influence of OCTN1/2 variants within the IBD5 locus on disease susceptibility and growth indices in early onset inflammatory bowel disease. Gut. 2006; 55:1114–1123.
Article131. Noble CL, Nimmo ER, Drummond H, et al. The contribution of OCTN1/2 variants within the IBD5 locus to disease susceptibility and severity in Crohn's disease. Gastroenterology. 2005; 129:1854–1864.
Article132. Potocnik U, Ferkolj I, Glavac D, Dean M. Polymorphisms in multidrug resistance 1 (MDR1) gene are associated with refractory Crohn disease and ulcerative colitis. Genes Immun. 2004; 5:530–539.
Article133. Katz KD, Hollander D, Vadheim CM, et al. Intestinal permeability in patients with Crohn's disease and their healthy relatives. Gastroenterology. 1989; 97:927–931.
Article134. Arnott ID, Kingstone K, Ghosh S. Abnormal intestinal permeability predicts relapse in inactive Crohn disease. Scand J Gastroenterol. 2000; 35:1163–1169.135. Wyatt J, Vogelsang H, Hübl W, Waldhöer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet. 1993; 341:1437–1439.136. Tsuji M, Suzuki K, Kinoshita K, Fagarasan S. Dynamic interactions between bacteria and immune cells leading to intestinal IgA synthesis. Semin Immunol. 2008; 20:59–66.
Article137. Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006; 169:1901–1909.138. Howell SJ, Wilk D, Yadav SP, Bevins CL. Antimicrobial polypeptides of the human colonic epithelium. Peptides. 2003; 24:1763–1770.
Article139. Tollin M, Bergman P, Svenberg T, et al. Antimicrobial peptides in the first line defence of human colon mucosa. Peptides. 2003; 24:523–530.
Article140. Sahl HG, Pag U, Bonness S, et al. Mammalian defensins: structures and mechanism of antibiotic activity. J Leukoc Biol. 2005; 77:466–475.
Article141. Hale JD, Hancock RE. Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Expert Rev Anti Infect Ther. 2007; 5:951–959.
Article142. Wehkamp J, Harder J, Weichenthal M, et al. Inducible and constitutive beta-defensins are differentially expressed in Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2003; 9:215–223.143. Schauber J, Rieger D, Weiler F, et al. Heterogeneous expression of human cathelicidin hCAP18/LL-37 in inflammatory bowel diseases. Eur J Gastroenterol Hepatol. 2006; 18:615–621.
Article144. Kobayashi KS, Chamaillard M, Ogura Y, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005; 307:731–734.
Article145. Wehkamp J, Harder J, Weichenthal M, et al. NOD2 (CARD15) mutations in Crohn's disease are associated with diminished mucosal alpha-defensin expression. Gut. 2004; 53:1658–1664.146. Wehkamp J, Salzman NH, Porter E, et al. Reduced Paneth cell alpha-defensins in ileal Crohn's disease. Proc Natl Acad Sci USA. 2005; 102:18129–18134.147. Fellermann K, Stange DE, Schaeffeler E, et al. A chromosome 8 gene-cluster polymorphism with low human beta-defensin 2 gene copy number predisposes to Crohn disease of the colon. Am J Hum Genet. 2006; 79:439–448.
Article148. Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001; 2:361–367.
Article149. Niess JH, Brand S, Gu X, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005; 307:254–258.150. Pulendran B, Tang H, Denning TL. Division of labor, plasticity, and crosstalk between dendritic cell subsets. Curr Opin Immunol. 2008; 20:61–67.
Article151. Rimoldi M, Chieppa M, Salucci V, et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005; 6:507–514.
Article152. Zoumpopoulou G, Tsakalidou E, Dewulf J, Pot B, Grangette C. Differential crosstalk between epithelial cells, dendritic cells and bacteria in a co-culture model. Int J Food Microbiol. 2009; 131:40–51.
Article153. Hoarau C, Martin L, Faugaret D, et al. Supernatant from bifidobacterium differentially modulates transduction signaling pathways for biological functions of human dendritic cells. PLoS One. 2008; 3:2753.
Article154. Foligne B, Zoumpopoulou G, Dewulf J, et al. A key role of dendritic cells in probiotic functionality. PLoS One. 2007; 2:313.
Article155. Niess JH, Leithäuser F, Adler G, Reimann J. Commensal gut flora drives the expansion of proinflammatory CD4 T cells in the colonic lamina propria under normal and inflammatory conditions. J Immunol. 2008; 180:559–568.
Article156. Gaboriau-Routhiau V, Rakotobe S, Lecuyer E, et al. The key role of segmented filamentous bacteria in the coordinate maturation of gut helper T cell responses. Immunity. 2009; 31:677–689.157. Ivanov II, Frutos Rde L, Manel N, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008; 4:337–349.
Article158. Chow J, Mazmanian SK. Getting the bugs out of the immune system: do bacterial microbiota “fix” intestinal T cell responses? Cell Host Microbe. 2009; 5:8–12.
Article159. Atarashi K, Nishimura J, Shima T, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008; 455:808–812.
Article160. Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008; 453:620–625.
Article161. Himmel ME, Hardenberg G, Piccirillo CA, Steiner TS, Levings MK. The role of T-regulatory cells and Toll-like receptors in the pathogenesis of human inflammatory bowel disease. Immunology. 2008; 125:145–153.
Article162. Torii A, Torii S, Fujiwara S, et al. Lactobacillus Acidophilus strain L-92 regulates the production of Th1 cytokine as well as Th2 cytokines. Allergol Int. 2007; 56:293–301.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Intestinal Microbiota in Inflammatory Bowel Diseases
- Role of Gut Microbiota-Gut Hormone Axis in the Pathophysiology of Functional Gastrointestinal Disorders
- Gut Microbiota in Inflammatory Bowel Disease
- Microbial Modulation in Inflammatory Bowel Diseases
- Is there a potential role of fecal microbiota transplantation in the treatment of inflammatory bowel disease?