Korean J Gastroenterol.
2013 Jan;61(1):37-41. 10.4166/kjg.2013.61.1.37.
A Case of Crohn's Disease Having Normal Delivery after Infliximab Treatment during Early Pregnancy
- Affiliations
-
- 1Department of Internal Medicine, Eulji General Hospital, Eulji University School of Medicine, Seoul, Korea. pys1109@eulji.ac.kr
- 2Department of Obstetrics & Gynecology, Eulji General Hospital, Eulji University School of Medicine, Seoul, Korea.
- 3Department of Pathology, Eulji General Hospital, Eulji University School of Medicine, Seoul, Korea.
- KMID: 1775785
- DOI: http://doi.org/10.4166/kjg.2013.61.1.37
Abstract
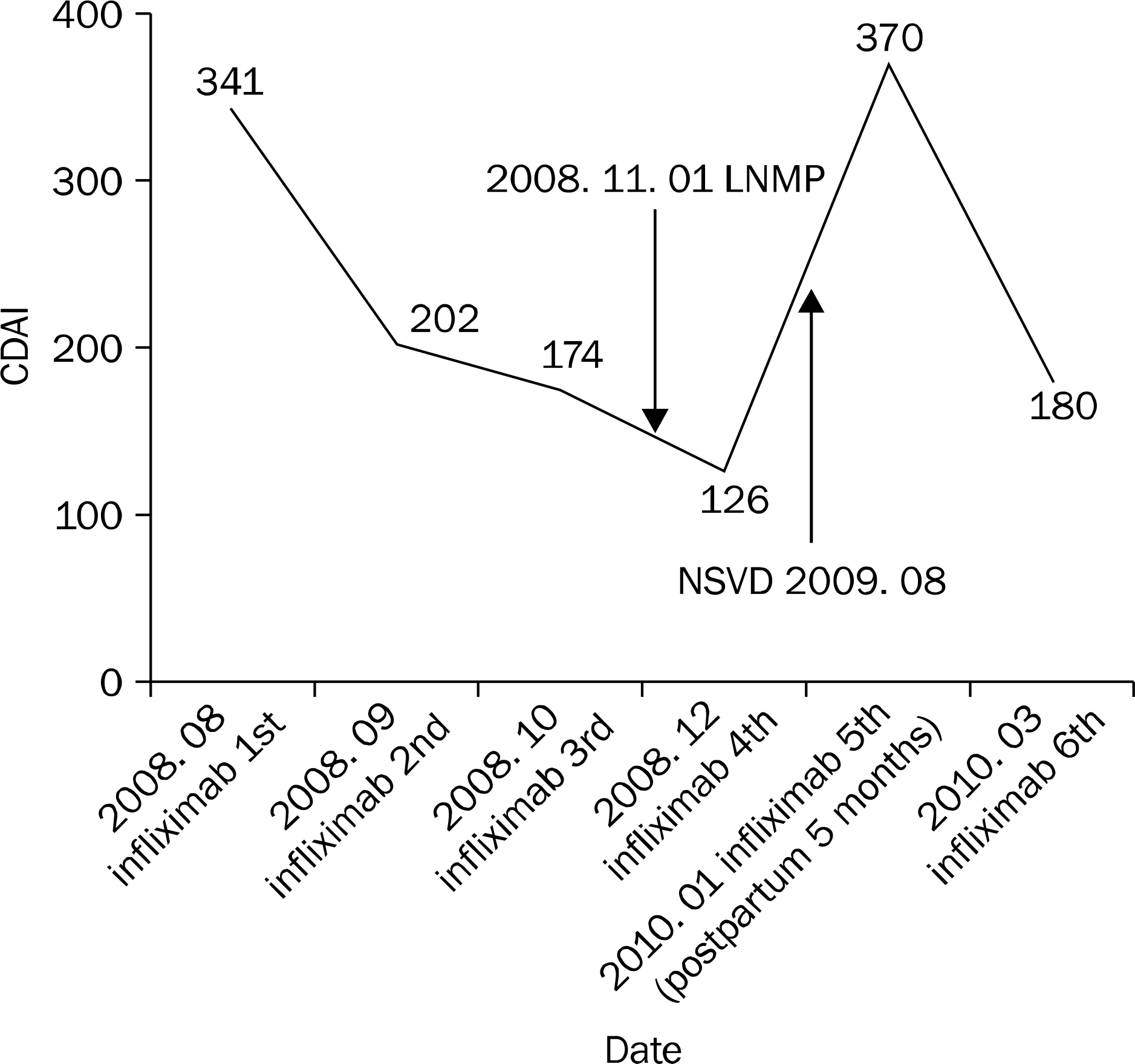
- Infliximab is a chimeric IgG1 monoclonal antibody to tumor necrosis factor (TNF)-alpha used in the treatment of steroid refractory or dependent Crohn's disease (CD). Patients with active CD are more likely to experience stillbirth, preterm labor, or small for gestational aged babies. The safety of administering infliximab in pregnant patients is not well documented. A 25-year-old woman, who was diagnosed with small bowel CD three years ago, was admitted to our hospital due to the aggravation of abdominal pain. She had been treated with mesalazine, azathioprine and intermittent steroid for three years. After admission, she did not respond to steroid therapy, we decided to try infliximab. After the administration of infliximab, epigastric pain was relived and Crohn's disease activity index score decreased significantly. However after the fourth infusion of infliximab, the patient became aware that she was ten gestational weeks old pregnancy state After then, infliximab was stopped and maintained by mesalazine. The patient gave birth to a healthy baby via normal vaginal delivery without the recurrence of CD. This case suggests that infliximab administration is safe during the early period of pregnancy. Thus, we report this case with a review of literature.
Keyword
MeSH Terms
-
Adult
Anti-Inflammatory Agents, Non-Steroidal/*therapeutic use
Antibodies, Monoclonal/*therapeutic use
Capsule Endoscopy
Colon, Sigmoid/pathology
Crohn Disease/*drug therapy/pathology
Female
Humans
Infant, Newborn
Mesalamine/therapeutic use
Pregnancy
Severity of Illness Index
Term Birth
Tomography, X-Ray Computed
Anti-Inflammatory Agents, Non-Steroidal
Antibodies, Monoclonal
Mesalamine
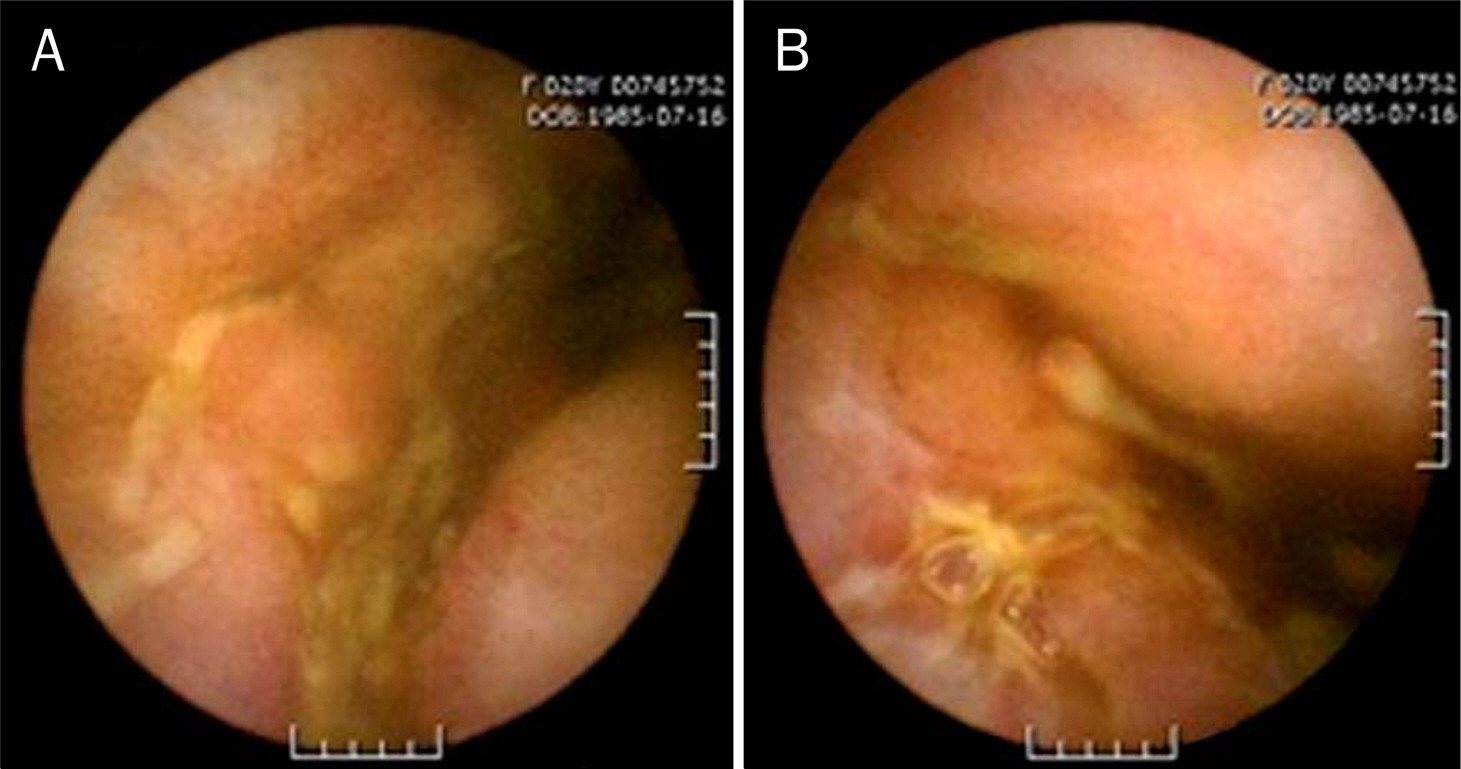
Figure
Reference
-
References
1. Yang SK, Yun S, Kim JH, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986–2005: a KASID study. Inflamm Bowel Dis. 2008; 14:542–549.
Article2. Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998; 115:182–205.
Article3. Hanauer SB, Feagan BG, Lichtenstein GR, et al. ACCENT I Study Group. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002; 359:1541–1549.
Article4. Lee SH. Use of TNF inhibitor in particular clinical settings. J Korean Rheum Assoc. 2009; 16:264–270.
Article5. Papa A, Mocci G, Bonizzi M, et al. Use of infliximab in particular clinical settings: management based on current evidence. Am J Gastroenterol. 2009; 104:1575–1586.
Article6. Habal FM, Ravindran NC. Management of inflammatory bowel disease in the pregnant patient. World J Gastroenterol. 2008; 14:1326–1332.
Article7. Kim WH. Inflammatory bowel disease and pregnancy. Intes Res. 2003; 2:141–158.8. Alstead EM, Nelson-Piercy C. Inflammatory bowel disease in pregnancy. Gut. 2003; 52:159–161.
Article9. Hudson M, Flett G, Sinclair TS, Brunt PW, Templeton A, Mowat NA. Fertility and pregnancy in inflammatory bowel disease. Int J Gynaecol Obstet. 1997; 58:229–237.
Article10. Park YS. Support good quality of life. Intes Res. 2010; 8(Suppl 2):103–110.11. Scallon BJ, Moore MA, Trinh H, Knight DM, Ghrayeb J. Chimeric anti-TNF-alpha monoclonal antibody cA2 binds recombinant transmembrane TNF-alpha and activates immune effector functions. Cytokine. 1995; 7:251–259.12. Kwan LY, Mahadevan U. Inflammatory bowel disease and pregnancy: an update. Expert Rev Clin Immunol. 2010; 6:643–657.
Article13. Lichtenstein G, Cohen RD, Fegan BG, et al. Safety of infliximab in Crohn's disease: data from 5000-patients TREAT registry. Gasteroenterology. 2004; 126(Suppl 2):A54.14. Katz JA, Antoni C, Keenan GF, Smith DE, Jacobs SJ, Lichtenstein GR. Outcome of pregnancy in women receiving infliximab for the treatment of Crohn's disease and rheumatoid arthritis. Am J Gastroenterol. 2004; 99:2385–2392.
Article15. Mahadevan U, Kane S, Sandborn WJ, et al. Intentional infliximab use during pregnancy for induction or maintenance of remission in Crohn's disease. Aliment Pharmacol Ther. 2005; 21:733–738.
Article16. Schnitzler F, Fidder H, Ferrante M, et al. Outcome of pregnancy in women with inflammatory bowel disease treated with anti-tumor necrosis factor therapy. Inflamm Bowel Dis. 2011; 17:1846–1854.
Article17. Vasiliauskas EA, Church JA, Silverman N, Barry M, Targan SR, Dubinsky MC. Case report: evidence for transplacental transfer of maternally administered infliximab to the newborn. Clin Gastroenterol Hepatol. 2006; 4:1255–1258.
Article18. Cheent K, Nolan J, Shariq S, Kiho L, Pal A, Arnold J. Case Report: Fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn's disease. J Crohns Colitis. 2010; 4:603–605.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Factors Affecting Surgical Treatment With Infliximab Therapy in Perianal Fistula With Crohn Disease
- Recent Trends of Infliximab Treatment for Crohn's Disease
- A Case of Infliximab-induced Psoriasis in Treatment of Ankylosing Spondylitis
- Change in the treatment strategy for pediatric Crohn's disease
- Adalimumab or infliximab: which is better for perianal fistula in Crohn's disease?