J Korean Acad Periodontol.
2009 Jun;39(2):129-138. 10.5051/jkape.2009.39.2.129.
Clinical study of guided bone regeneration of extracted socket with PLA/PGA membrane and silk fibroin membrane
- Affiliations
-
- 1Department of Periodontology, School of Dentistry, Wonkwang University, Korea. periohs@wonkwang.ac.kr
- 2Department of Periodontology, School of Dentistry, Seoul National University, Korea.
- 3NIBEC Inc., Korea.
- KMID: 1733730
- DOI: http://doi.org/10.5051/jkape.2009.39.2.129
Abstract
-
PURPOSE: This study was designed to compare the bond regeneratiom effects of treatment using silk fibroin membrane ( Nanogide-S (R)) resorbable barrier with control group treated by polyactic acid / polylacticglycolic acid membrane(Biomesh (R) )
METHODS
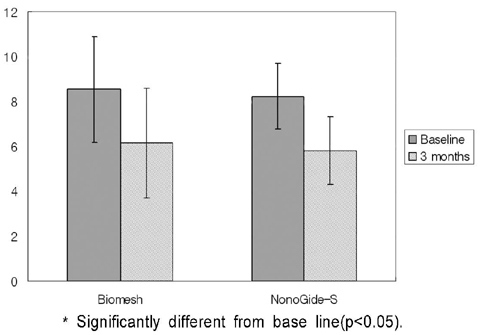
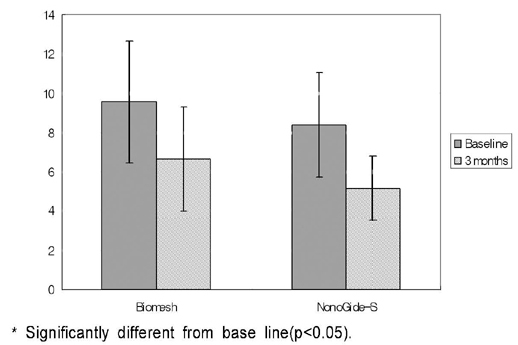
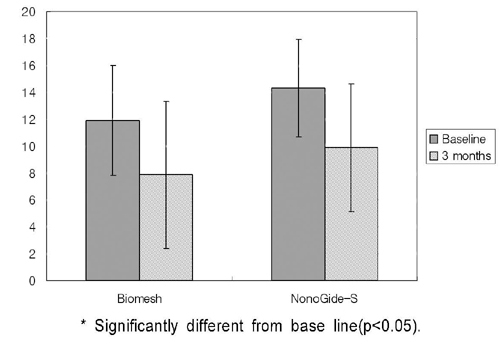
44 severe bone loss on extraction socket from 44 patients were used in this study. In experimental group 22 sites of them were treated by silk fibrin membrane as and the other 22 sites were treated by polyactic acid/ polylacticglycolic acid membrane as a control group. Clinical parameters including recovered bone width, length and radiographic parameter of vertical length were evlauated at base line and 3 months after surgery.
RESULTS
1) Severe bone width, length was significantlly decreased in two group. 2) Bone width, length was significantlly decreased in two group. 3) Decreased bone width, length and radiographic examination differences between group.
CONCLUSIONS
On the basis of these results, silk fibrin resorbable membrane has similar bone regeneration ability to polyactic acid / polylacticglycolic acid membrane in guided bone regeneration for severe bone loss defect on extraction socket.
MeSH Terms
Figure
Reference
-
1. Pfeifer J, Swol RL, Ellinger R, et al. Epithelial exclusion and tissue regeneration using a collagen membrane barrier in chronic periodontal defects. A historical study. Int J Periodontics Restorative Dent. 1989. 9:263.2. Dahlin C, Lindhe A, Gottlow J, Nyman S, et al. "Healing of maxillary and mandibular defects by a mem brane technique: an experimental study in monkeys". Scand J Plast Reconst Hand Surg. 1990. 24:13–19.
Article3. Dahlin C, Lindhe J, Gottlow S, Nyman S, et al. "Healing of bone defects by guided tissue regeneration". J Plast Reconstr Surg. 1988. 81:672–676.
Article4. Kim M, Kim JH, Lee JY, et al. Effect of bone mineral with or without collagen membrane in ridge dehiscence defects following premolar extraction. In Vivo. 2008. 22(2):231–236.5. Wallace S, Froum J, Cho SC, et al. Sinus augmen tation utilizing anorganic bovine bone(Bio-Oss) with absorbable and nonabsorbable membranes placed over the lateral window: histomorphometric and clinical analyses. Int J Periodontics Restorative Dent. 2005. 25:551–559.6. Schulz AJ, Grager AH, et al. Guided tissue regeneration using on absorbable membrane (poly glactin 910) and osseous grafting. Int J Periodont. 1990. 10:8.7. Balshi TJ, Hernadez OD, Culter RH, Hertzog CF, et al. Treatment of osseous defects using vicryl mesh (poly glactin 910) and the Braenemark implant, A case report. Int J Oral Max-Fac Implants. 1991. 6:87.8. Tal H, Pitaru S, et al. Formation of new periodontal attachment apparatus after experimental root isolation with collagen membranes in the dog, . Int J Periodontics Restorative Dent. 1992. 12(3):231–242.9. Stavropoulos A, Sculean A, Karring T. GTR treatment of intrabony defects with PLA/PGA copolymer or collagen bioresorbable membranes in combination with deproteinized bovine bone(Bio-Oss). Clin Oral Investig. 2004. 8(4):226–232.
Article10. Jung RE, Glauser R, Scharer P, et al. Effect of rhBMP-2 on guided bone regeneration in humans. Clin Oral Implants Res. 2003. 14(5):556–568.
Article11. Takeishi H, Irie K, Okuda K, et al. Molded bone augmentation by a combination of barrier membrane and recombinant human bone morphogenetic protein-2. Oral Dis. 2001. 7(5):281–286.
Article12. Um IC, Kweon HY, Park YH, Hudson S, et al. Structural characteristics and properties of the regenerated silk fibroin prepared from formic acid. Int J Biol Macromol. 2001. 29:91–99.
Article13. Um IC, Park YH. Morphology of Silk Fibroin / Poly( vinyl alcohol) Blend Film. J of Korean Society of Sericultural Science. 1998. 40(7):169–175.14. Choi HK, Nahm JH. Preparation and Structural Characterization of Silk Fibroin Powder and Film. J of Korean Society of Sericultural Science. 1995. 37(12):142–153.15. Minoura N, Aiba S, Higuchi M, et al. Attachment and growth of fibroblast cells on silk fibroin. Biochem Biophys Res Commun. 1995. 208:511–516.
Article16. Minoura N, Tsukada M, Nagura M, et al. Fine structure and oxygen permeability of silk fibroin membrane treated with methanol. Polymer. 1990. 31:265–269.
Article17. Singer AJ, Clark RA, et al. Cutaneous wound healing. N Engl J Med. 1999. 341:738–746.
Article18. Rios CN, Skoracki RJ, Miller MJ, et al. In vivo Bone formation in silk fibroin and chitosan blend scaffolds via ectopically grafted periosteum as a cell source: a pilot study. Tissue Enq Part A. 2009. 02. 18.19. Efficacy of polarized hydroxyapatite and silk fibroin composite dressing gel on epidermal recovery from full-thickness skin wounds. J Biomed Mater Res B Appl Biomter. 2009. 02. 11.20. Stahl SS. Morphology and healing pattern of human interdental gingivae. J Am Dent Assoc. 1963. 67:48–53.21. Gottlow J, Nyman S, Lindhe J, Karring T, Wennstrom J, et al. New attachment formation in the human periodontium by guided tissue regeneration. Case reports. J Clin Periodontol. 1986. 13(6):604–616.
Article22. Aukhil I, Pettersson E, Suggs C, et al. Guided tissue regeneration. An experimental procedure in beagle dogs. J Periodontol. 1986. 57(12):727–734.
Article23. Jung RE, Hälg GA, Thoma DS, et al. A randomized, controlled clinical trial to evaluate a new membrane for guided bone regeneration around dental implants. Clin Oral Implants Res. 2009. 02. 20(2):162–168.
Article24. Lee EJ, Shin DS, Kim HE, et al. Membrane of hybrid chitosan-silica xerogel for guided bone regeneration. Biomaterials. 2009. 02. 30(5):743–750.
Article25. Kinoshita Y, Matsuo M, Todoki K, et al. Alveolar bone regeneration using absorbable poly(L-lactide-co-epsilon-caprolactone)/beta-tricalcium phosphate membrane and gelatin sponge incorporating basic fibroblast growth factor. Int J Oral Maxillofac Surg. 2008. 03. 37(3):275–281.
Article26. Oh SH, Kim JH, Kim JM, et al. Asymmetrically porous PLGA/Pluronic F127 membrane for effective guided bone regeneration. J Biomater Sci Polym Ed. 2006. 17(12):1375–1387.
Article27. Fleisher N, Waa H, Bloom A, et al. Regeneration of lost attachment apparatus in the dog using Vicryl absorbable mesh(Polylactin 910®). Int J Periodontics Restorative Dent. 1988. 2:45–55.28. Zellin G, Linde A, et al. Healing of mandibular defects with different biodegradable and nonbiodegradable membranes: an experimental study in rats. Biomaterials. 1995. 16:601–609.
Article29. Minoura N, Aiba S, Higuchi M, et al. Attachment and growth of fibroblast cells on silk fibroin. Biochem Biophys Res Commun. 1995. 03. 17. 208(2):511–516.
Article30. Schropp L, Wenzel A, Kostopoulos L, et al. Bone healing and soft tissue contour changes following single tooth extraction: A clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003. 23:313–323.31. Nevin M, Mellonig J, et al. "Enhancement of the damaged edentulous ridge to receive dental implant: A combination of allogrft and the Gore-Tex membrane". Int J Periodontics Restorative Dent. 1992. 12:97–111.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A comparative study for guided bone regeneration of silk fibroin nanomembrane(NanoGide-S(TM))
- Membranes for the Guided Bone Regeneration
- Cell attachment and proliferation of osteoblast-like MG63 cells on silk fibroin membrane for guided bone regeneration
- Effects of the Guided Tissue Regeneration Using Polylactic/Polyglycolic Copolymer Membrane in the Furcation Involvement
- The Effects of Tetracycline-loaded Silk Fibroin Membrane on Guided Bone Regeneration in a Rabbit Calvarial Defect Model