Clin Exp Vaccine Res.
2014 Jul;3(2):202-211. 10.7774/cevr.2014.3.2.202.
Immunogenicity and efficacy of a plasmid DNA rabies vaccine incorporating Myd88 as a genetic adjuvant
- Affiliations
-
- 1Department of Neurovirology, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, India. mshampur@gmail.com
- KMID: 1730625
- DOI: http://doi.org/10.7774/cevr.2014.3.2.202
Abstract
- PURPOSE
Myeloid differentiation factor 88 (Myd88), a ubiquitous Toll-like receptor adaptor molecule, has been reported to play important roles in B cell responses to infections and vaccination. The present study evaluated the effects of genetic adjuvanting with Myd88 on the immune responses to a plasmid DNA rabies vaccine.
MATERIALS AND METHODS
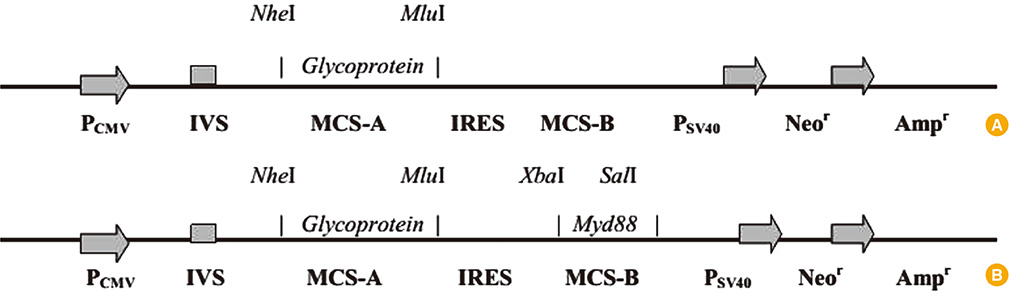
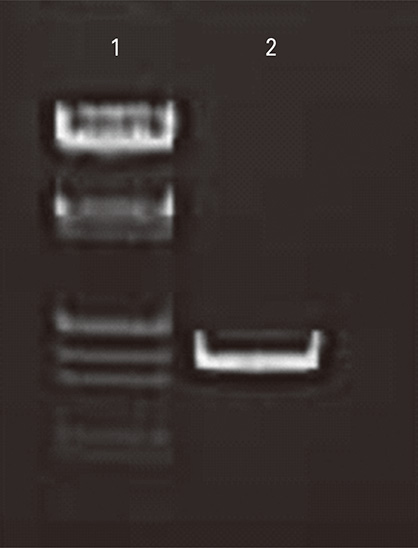
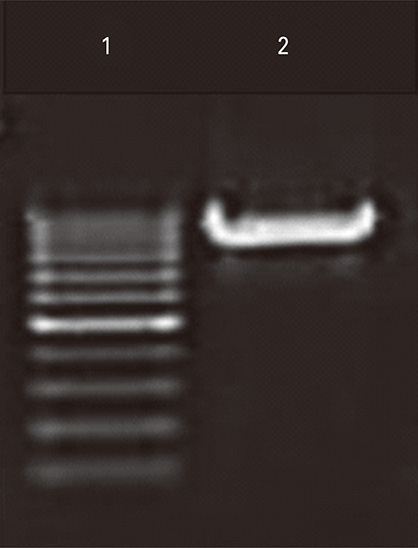
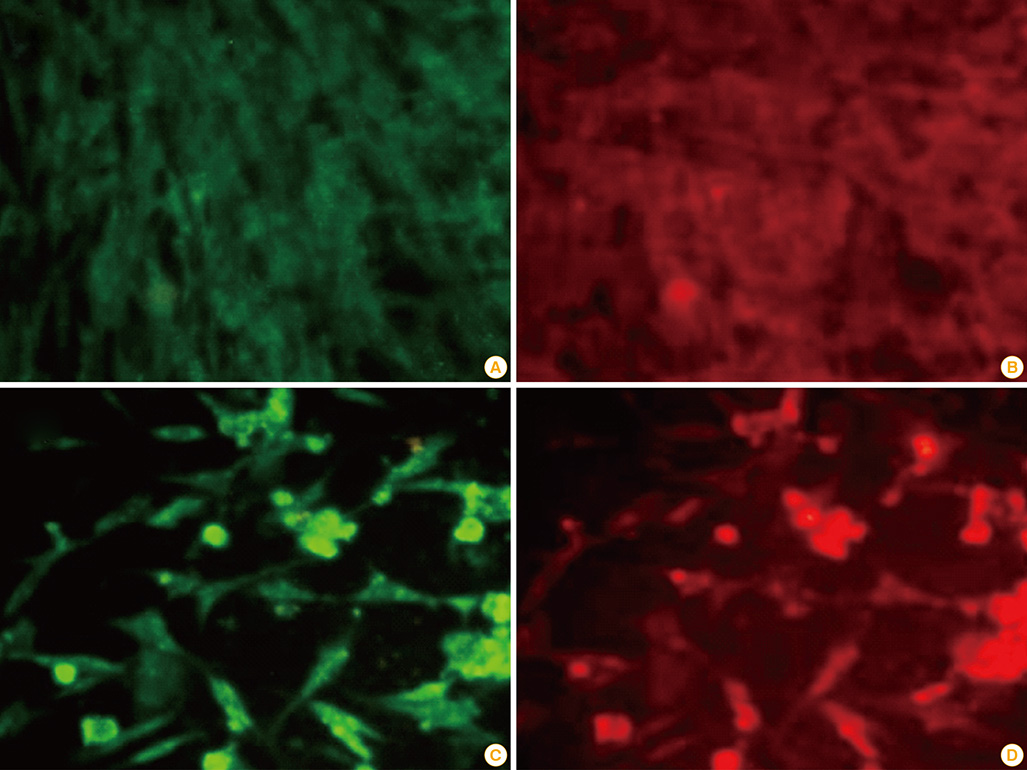
Plasmids encoding rabies glycoprotein alone (pIRES-Rgp) or a fragment of Myd88 gene in addition (pIRES-Rgp-Myd) were constructed and administered intramuscularly or intrademally in Swiss albino mice (on days 0, 7, and 21). Rabies virus neutralizing antibody (RVNA) titres were estimated in the mice sera on days 14 and 28 by rapid fluorescent focus inhibition test. The protective efficacy of the constructs was evaluated by an intracerebral challenge with challenge virus standard virus on day 35.
RESULTS
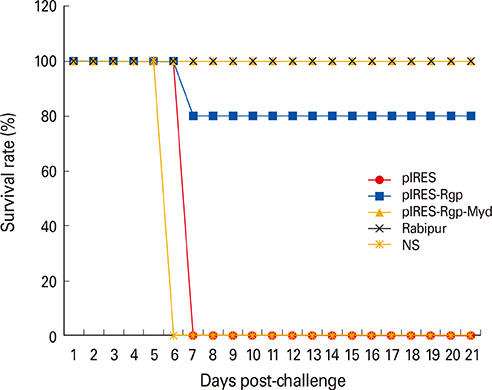
Co-expression of Myd88 increased RVNA responses to pIRES-Rgp by 3- and 2-folds, following intramuscular and intradermal immunization, respectively. pIRES-Rgp protected 80% of the mice following intramuscular and intradermal immunizations, while pIRES-Rgp-Myd afforded 100% protection following similar administrations.
CONCLUSION
Genetic adjuvanting with Myd88 enhanced the RVNA responses and protective efficacy of a plasmid DNA rabies vaccine. This strategy might be useful for rabies vaccination of canines in the field, and needs further evaluation.
MeSH Terms
-
Animals
Antibodies, Neutralizing
DNA*
Glycoproteins
Immunization
Mice
Myeloid Differentiation Factor 88
Plasmids*
Rabies
Rabies Vaccines*
Rabies virus
Toll-Like Receptors
Vaccination
Vaccines, DNA
Antibodies, Neutralizing
DNA
Glycoproteins
Myeloid Differentiation Factor 88
Rabies Vaccines
Toll-Like Receptors
Vaccines, DNA
Figure
Cited by 1 articles
-
Improvement of DNA vaccination by adjuvants and sophisticated delivery devices: vaccine-platforms for the battle against infectious diseases
Thomas Grunwald, Sebastian Ulbert
Clin Exp Vaccine Res. 2015;4(1):1-10. doi: 10.7774/cevr.2015.4.1.1.
Reference
-
1. Knobel DL, Cleaveland S, Coleman PG, et al. Re-evaluating the burden of rabies in Africa and Asia. Bull World Health Organ. 2005; 83:360–368.2. Rupprecht CE, Hanlon CA, Hemachudha T. Rabies re-examined. Lancet Infect Dis. 2002; 2:327–343.
Article3. World Health Organization. Guidelines for dog rabies control. VH/83.43 Rev. 1. Geneva: WHO;1986.4. Bahloul C, Taieb D, Diouani MF, et al. Field trials of a very potent rabies DNA vaccine which induced long lasting virus neutralizing antibodies and protection in dogs in experimental conditions. Vaccine. 2006; 24:1063–1072.
Article5. Ullas PT, Desai A, Madhusudana SN. Rabies DNA vaccines: current status and future. World J Vaccines. 2012; 2:36–45.
Article6. Barry ME, Pinto-Gonzalez D, Orson FM, McKenzie GJ, Petry GR, Barry MA. Role of endogenous endonucleases and tissue site in transfection and CpG-mediated immune activation after naked DNA injection. Hum Gene Ther. 1999; 10:2461–2480.
Article7. Ulmer JB, Wahren B, Liu MA. Gene-based vaccines: recent technical and clinical advances. Trends Mol Med. 2006; 12:216–222.
Article8. Ullas PT, Madhusudana SN, Desai A, et al. Enhancement of immunogenicity and efficacy of a plasmid DNA rabies vaccine by nanoformulation with a fourth-generation amine-terminated poly (ether imine) dendrimer. Int J Nanomedicine. 2014; 9:627–634.9. Singh M, Briones M, Ott G, O'Hagan D. Cationic microparticles: a potent delivery system for DNA vaccines. Proc Natl Acad Sci U S A. 2000; 97:811–816.
Article10. Lodmell DL, Parnell MJ, Bailey JR, Ewalt LC, Hanlon CA. Rabies DNA vaccination of non-human primates: post-exposure studies using gene gun methodology that accelerates induction of neutralizing antibody and enhances neutralizing antibody titers. Vaccine. 2002; 20:2221–2228.
Article11. Margalith M, Vilalta A. Sustained protective rabies neutralizing antibody titers after administration of cationic lipid-formulated pDNA vaccine. Genet Vaccines Ther. 2006; 4:2.12. Bodles-Brakhop AM, Heller R, Draghia-Akli R. Electroporation for the delivery of DNA-based vaccines and immunotherapeutics: current clinical developments. Mol Ther. 2009; 17:585–592.
Article13. Kaur M, Saxena A, Rai A, Bhatnagar R. Rabies DNA vaccine encoding lysosome-targeted glycoprotein supplemented with Emulsigen-D confers complete protection in preexposure and postexposure studies in BALB/c mice. FASEB J. 2010; 24:173–183.
Article14. Xiang SD, Selomulya C, Ho J, Apostolopoulos V, Plebanski M. Delivery of DNA vaccines: an overview on the use of biodegradable polymeric and magnetic nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010; 2:205–218.
Article15. Nawwab Al-Deen FM, Selomulya C, Kong YY, et al. Design of magnetic polyplexes taken up efficiently by dendritic cell for enhanced DNA vaccine delivery. Gene Ther. 2014; 21:212–218.
Article16. Kobiyama K, Jounai N, Aoshi T, et al. Innate immune signaling by, and genetic adjuvants for DNA vaccination. Vaccines. 2013; 1:278–292.
Article17. Wales J, Andreakos E, Feldmann M, Foxwell B. Targeting intracellular mediators of pattern-recognition receptor signalling to adjuvant vaccination. Biochem Soc Trans. 2007; 35(Pt 6):1501–1503.
Article18. Xiang Z, Ertl HC. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity. 1995; 2:129–135.
Article19. Geissler M, Gesien A, Tokushige K, Wands JR. Enhancement of cellular and humoral immune responses to hepatitis C virus core protein using DNA-based vaccines augmented with cytokine-expressing plasmids. J Immunol. 1997; 158:1231–1237.20. Tsuji T, Hamajima K, Ishii N, et al. Immunomodulatory effects of a plasmid expressing B7-2 on human immunodeficiency virus-1-specific cell-mediated immunity induced by a plasmid encoding the viral antigen. Eur J Immunol. 1997; 27:782–787.
Article21. Xiang ZQ, He Z, Wang Y, Ertl HC. The effect of interferon-gamma on genetic immunization. Vaccine. 1997; 15:896–898.22. Sin JI, Kim JJ, Ugen KE, Ciccarelli RB, Higgins TJ, Weiner DB. Enhancement of protective humoral (Th2) and cell-mediated (Th1) immune responses against herpes simplex virus-2 through co-delivery of granulocyte-macrophage colony-stimulating factor expression cassettes. Eur J Immunol. 1998; 28:3530–3540.
Article23. Pinto AR, Reyes-Sandoval A, Ertl HC. Chemokines and TRANCE as genetic adjuvants for a DNA vaccine to rabies virus. Cell Immunol. 2003; 224:106–113.
Article24. Bramson JL, Dayball K, Hall JR, et al. Super-activated interferon-regulatory factors can enhance plasmid immunization. Vaccine. 2003; 21:1363–1370.
Article25. O'Neill LA, Golenbock D, Bowie AG. The history of Toll-like receptors: redefining innate immunity. Nat Rev Immunol. 2013; 13:453–460.26. Genestier L, Taillardet M, Mondiere P, Gheit H, Bella C, Defrance T. TLR agonists selectively promote terminal plasma cell differentiation of B cell subsets specialized in thymus-independent responses. J Immunol. 2007; 178:7779–7786.
Article27. O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007; 7:353–364.28. Rubtsov AV, Swanson CL, Troy S, Strauch P, Pelanda R, Torres RM. TLR agonists promote marginal zone B cell activation and facilitate T-dependent IgM responses. J Immunol. 2008; 180:3882–3888.
Article29. Krieg AM. Toll-free vaccines? Nat Biotechnol. 2007; 25:303–305.
Article30. Takeshita F, Tanaka T, Matsuda T, et al. Toll-like receptor adaptor molecules enhance DNA-raised adaptive immune responses against influenza and tumors through activation of innate immunity. J Virol. 2006; 80:6218–6224.
Article31. Smith JS, Yager PA, Baer GM. A rapid reproducible test for determining rabies virus-neutralizing antibody. In : Meslin FX, Kaplan MM, Koprowski H, editors. Laboratory techniques in rabies. 4th ed. Geneva: World Health Organization;1996. p. 181–192.32. Mintzer MA, Simanek EE. Nonviral vectors for gene delivery. Chem Rev. 2009; 109:259–302.
Article33. Gururajan M, Jacob J, Pulendran B. Toll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsets. PLoS One. 2007; 2:e863.
Article34. Ha SA, Tsuji M, Suzuki K, et al. Regulation of B1 cell migration by signals through Toll-like receptors. J Exp Med. 2006; 203:2541–2550.
Article35. Guay HM, Andreyeva TA, Garcea RL, Welsh RM, Szomolanyi-Tsuda E. MyD88 is required for the formation of long-term humoral immunity to virus infection. J Immunol. 2007; 178:5124–5131.
Article36. von Bernuth H, Picard C, Jin Z, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008; 321:691–696.
Article37. Sheahan T, Morrison TE, Funkhouser W, et al. MyD88 is required for protection from lethal infection with a mouse-adapted SARS-CoV. PLoS Pathog. 2008; 4:e1000240.
Article38. Neves P, Lampropoulou V, Calderon-Gomez E, et al. Signaling via the MyD88 adaptor protein in B cells suppresses protective immunity during Salmonella typhimurium infection. Immunity. 2010; 33:777–790.
Article39. Kang SM, Yoo DG, Kim MC, et al. MyD88 plays an essential role in inducing B cells capable of differentiating into antibody-secreting cells after vaccination. J Virol. 2011; 85:11391–11400.
Article40. Kaur M, Rai A, Bhatnagar R. Rabies DNA vaccine: no impact of MHC class I and class II targeting sequences on immune response and protection against lethal challenge. Vaccine. 2009; 27:2128–2137.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunogenicity of an inactivated rabies vaccine for animals derived from the recombinant ERAGS strain
- The present and future of rabies vaccine in animals
- A single immunization with recombinant rabies virus (ERAG3G) confers complete protection against rabies in mice
- Codelivery of IL-7 Augments Multigenic HCV DNA Vaccine-induced Antibody as well as Broad T Cell Responses in Cynomolgus Monkeys
- A genetically modified rabies vaccine (ERAGS) induces protective immunity in dogs and cattle