Yonsei Med J.
2011 Jul;52(4):616-623. 10.3349/ymj.2011.52.4.616.
Teicoplanin Dosing Strategy for Treatment of Staphylococcus aureus in Korean Patients with Neutropenic Fever
- Affiliations
-
- 1Department of Medicine, Graduate School, Dongguk University, Seoul, Korea.
- 2Department of Pharmacology, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. symonlee@catholic.ac.kr
- KMID: 1727994
- DOI: http://doi.org/10.3349/ymj.2011.52.4.616
Abstract
- PURPOSE
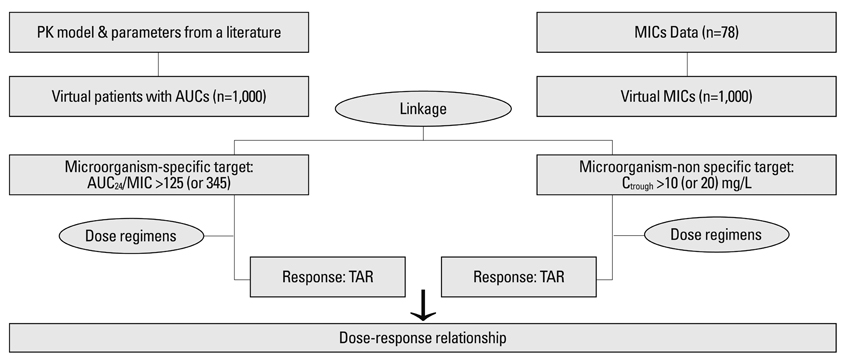
The present study was conducted to determine and compare the target attainment rate (TAR) between microorganism-nonspecific (Ctrough) and microorganism-specific (AUC24/MIC) targets over two weeks of teicoplanin administration according to several dose regimens for the treatment of Staphylococcus aureus in Korean patients with neutropenic fever.
MATERIALS AND METHODS
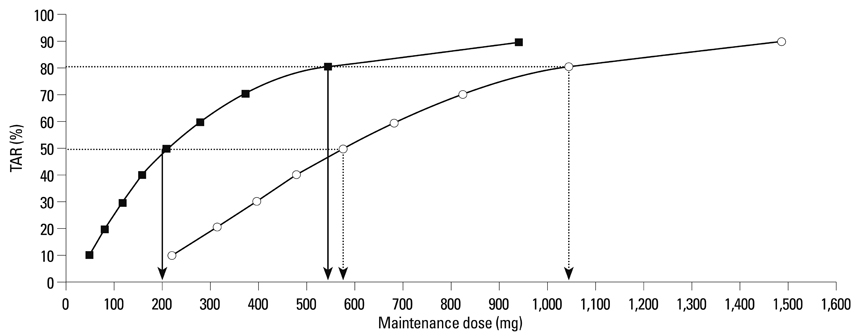
One thousand virtual concentrations were obtained for each dose using the population pharmacokinetic parameters of teicoplanin adopted from a published study. Simulation of 1,000 virtual MICs was performed using the MICs of 78 clinical isolates of S. aureus collected from a hospital in Korea. Thereafter, these simulated MICs were randomly allocated to 1,000 virtual patients in whom the TARs for AUC24/MIC >125 [or 345] and Ctrough >10 [or 20] mg/L were determined. The relationship of the maintenance dose with the steady-state TAR was predicted with respect to the AUC24/MIC >125 [or 345] using logistic analysis.
RESULTS
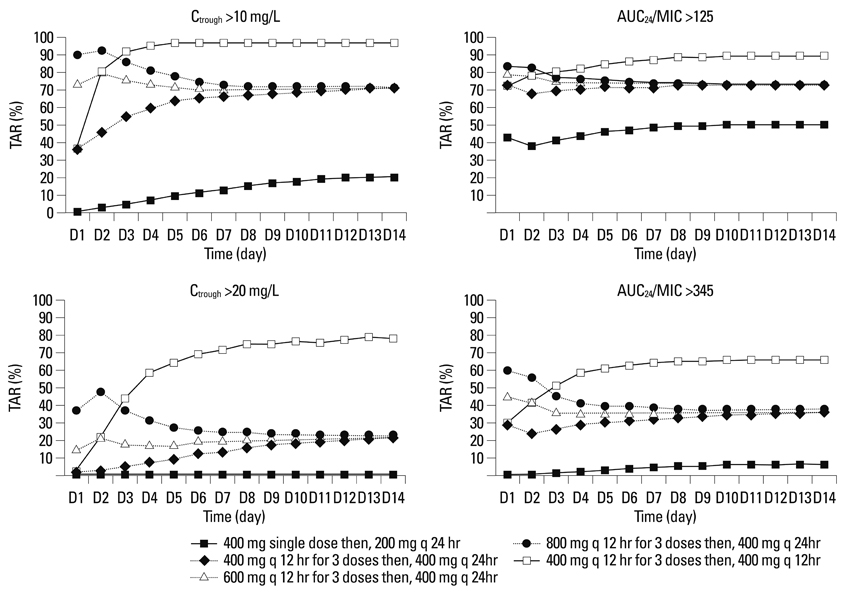
The standard dose regimen of teicoplanin showed TARs of about 70% [or 33%] and 70% [or 20%] at steady-state in cases with AUC24/MIC >125 [or 345] and Ctrough >10 [or 20] mg/L, respectively.
CONCLUSION
The current standard dose regimen was predicted to be insufficient to adequately treat S. aureus in Korean patients with neutropenic fever. To assure at least an 80% TAR in this population, dose adjustment of teicoplanin should be considered.
MeSH Terms
-
Anti-Bacterial Agents/administration & dosage/*pharmacology/therapeutic use
Computer Simulation
Dose-Response Relationship, Drug
Fever/drug therapy/microbiology
Humans
Microbial Sensitivity Tests
Neutropenia/drug therapy/microbiology
Republic of Korea
Staphylococcal Infections/drug therapy
Staphylococcus aureus/*drug effects
Teicoplanin/administration & dosage/*pharmacology/therapeutic use
Treatment Outcome
Figure
Reference
-
1. Park SH, Choi SM, Lee DG, Choi JH, Yoo JH, Lee JW, et al. Current trends of infectious complications following hematopoietic stem cell transplantation in a single center. J Korean Med Sci. 2006. 21:199–207.
Article2. Lee DG, Yim DS, Choi SM, Park SH, Yoo JH, Choi JH, et al. Efficacies of teicoplanin in patients with febrile neutropenia. Infect Chemother. 2004. 36:83–91.3. Wilson AP. Clinical pharmacokinetics of teicoplanin. Clin Pharmacokinet. 2000. 39:167–183.
Article4. MacGowan AP. Pharmacodynamics, pharmacokinetics, and therapeutic drug monitoring of glycopeptides. Ther Drug Monit. 1998. 20:473–477.
Article5. Harding I, MacGowan AP, White LO, Darley ES, Reed V. Teicoplanin therapy for Staphylococcus aureus septicaemia: relationship between pre-dose serum concentrations and outcome. J Antimicrob Chemother. 2000. 45:835–841.
Article6. MacGowan AP, White LO, Reeves D, Harding I. Retrospective review of serum teicoplanin concentrations in clinical trials and their relationship to clinical outcome. J Infect Chemother. 1996. 2:197–208.
Article7. Hyatt JM, McKinnon PS, Zimmer GS, Schentag JJ. The importance of pharmacokinetic/pharmacodynamic surrogate markers to outcome. Focus on antibacterial agents. Clin Pharmacokinet. 1995. 28:143–160.
Article8. Korting HC, Schäfer-Korting M. The benefit/risk ratio: a handbook for the rational use of potentially hazardous drugs. 1999. Boca Raton: CRC Press.9. Moise PA, Forrest A, Bhavnani SM, Birmingham MC, Schentag JJ. Area under the inhibitory curve and a pneumonia scoring system for predicting outcomes of vancomycin therapy for respiratory infections by Staphylococcus aureus. Am J Health Syst Pharm. 2000. 57:Suppl 2. S4–S9.
Article10. Craig WA. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect Dis Clin North Am. 2003. 17:479–501.
Article11. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Sixteenth informational supplement. Approved standard M100-S16. 2006. Wayne, PA: CLSI.12. Lortholary O, Tod M, Rizzo N, Padoin C, Biard O, Casassus P, et al. Population pharmacokinetic study of teicoplanin in severely neutropenic patients. Antimicrob Agents Chemother. 1996. 40:1242–1247.
Article13. Svetitsky S, Leibovici L, Paul M. Comparative efficacy and safety of vancomycin versus teicoplanin: systematic review and meta-analysis. Antimicrob Agents Chemother. 2009. 53:4069–4079.
Article14. Whitehouse T, Cepeda JA, Shulman R, Aarons L, Nalda-Molina R, Tobin C, et al. Pharmacokinetic studies of linezolid and teicoplanin in the critically ill. J Antimicrob Chemother. 2005. 55:333–340.
Article15. Gimenez F, Leblond V, Nguyen J, Serrand P, Binet JL, Grosset J, et al. Variations of teicoplanin concentrations in neutropenic patients. J Clin Pharm Ther. 1997. 22:187–190.
Article16. Schentag JJ. Antimicrobial management strategies for Gram-positive bacterial resistance in the intensive care unit. Crit Care Med. 2001. 29:N100–N107.
Article17. Pea F, Brollo L, Viale P, Pavan F, Furlanut M. Teicoplanin therapeutic drug monitoring in critically ill patients: a retrospective study emphasizing the importance of a loading dose. J Antimicrob Chemother. 2003. 51:971–975.
Article18. Lortholary O, Lefort A, Tod M, Chomat AM, Darras-Joly C, Cordonnier C. Pharmacodynamics and pharmacokinetics of antibacterial drugs in the management of febrile neutropenia. Lancet Infect Dis. 2008. 8:612–620.
Article19. Pea F, Viale P, Candoni A, Pavan F, Pagani L, Damiani D, et al. Teicoplanin in patients with acute leukaemia and febrile neutropenia: a special population benefiting from higher dosages. Clin Pharmacokinet. 2004. 43:405–415.20. Van Bambeke F. Glycopeptides in clinical development: pharmacological profile and clinical perspectives. Curr Opin Pharmacol. 2004. 4:471–478.
Article21. McKinnon PS, Davis SL. Pharmacokinetic and pharmacodynamic issues in the treatment of bacterial infectious diseases. Eur J Clin Microbiol Infect Dis. 2004. 23:271–288.
Article22. Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004. 43:925–942.
Article23. Stass H, Dalhoff A. The integrated use of pharmacokinetic and pharmacodynamic models for the definition of breakpoints. Infection. 2005. 33:Suppl 2. 29–35.
Article24. Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC, Craig WA, Billeter M, et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009. 49:325–327.
Article25. Kuti JL, Kiffer CR, Mendes CM, Nicolau DP. Pharmacodynamic comparison of linezolid, teicoplanin and vancomycin against clinical isolates of Staphylococcus aureus and coagulase-negative staphylococci collected from hospitals in Brazil. Clin Microbiol Infect. 2008. 14:116–123.
Article26. Weinbren M, Struthers K. Emergence of Staphylococcus aureus (MRSA) with reduced susceptibility to teicoplanin during therapy. J Antimicrob Chemother. 2002. 50:306–307.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Wound Infection Caused by Staphylococcus aureus with Decreased Susceptibility to Teicoplanin
- Methicillin-Resistant Staphylococcus aureus Endocarditis Involving Tricuspid Valve in Ventricular Septal Defect with Multiple Pulmonary Embolism
- Teicoplanin-induced Elevation of Plasma Creatine Phosphokinase in the Patient with Wound Infection: A case report
- Treatment Strategy for Staphylococcus aureus Bacteremia
- A Study of Antibiotic Susceptibility of Staphylococcus aureus in Bacterial Skin Infections