J Bacteriol Virol.
2014 Sep;44(3):269-273. 10.4167/jbv.2014.44.3.269.
Human Adenovirus Type 5 as a Delivery Vector is Not Neutralized in Field Serum Samples of Cattle, Pig, and Goat of Republic of Korea
- Affiliations
-
- 1Foot-and-Mouth Disease Division, Animal and Plant Quarantine Agency, Ministry of Agriculture, Food and Rural Affairs, Gyeonggi-do, Korea. beliefsk@korea.kr
- KMID: 1726977
- DOI: http://doi.org/10.4167/jbv.2014.44.3.269
Abstract
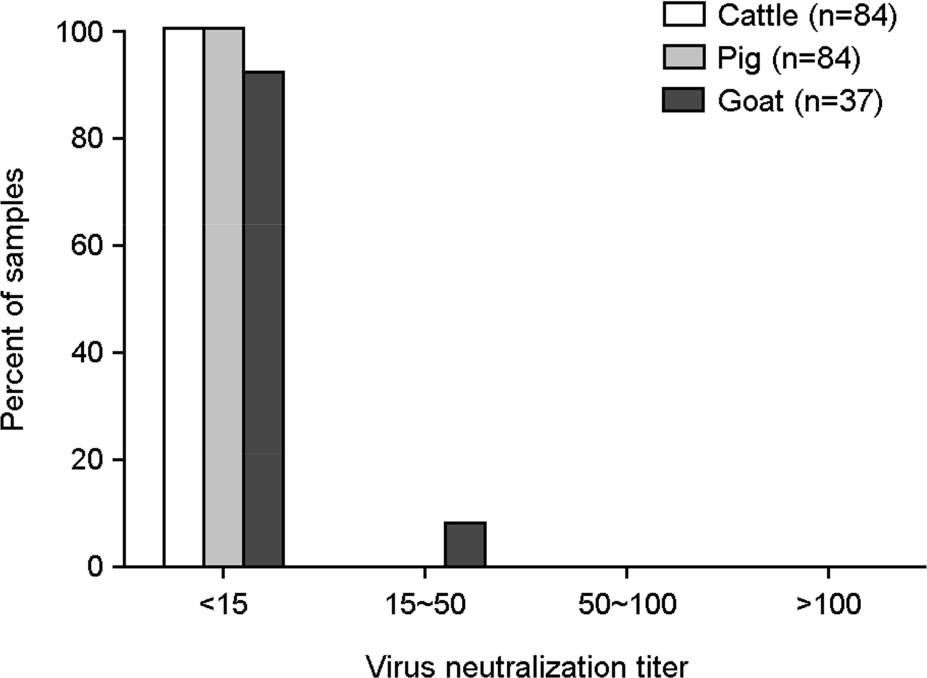
- Human adenovirus type 5 (hAd5) vectors have been demonstrated to be useful vehicles for gene expressions in animals. However, it has not been reported whether hAd5 transduction might be hampered in the sera of livestock animals in Republic of Korea. We collected 205 samples of livestock animals, such as pig (n=84), cattle (n=84), and goat (n=37) in Korea. The neutralizing antibody (NAb) titers to hAd5 virus were less than 15 in most of samples. Only 8% of goat samples had a NAb titer of 15 or 30. Thus, we showed that hAd5 virus was not neutralized in sera from cattle, pig, and goat, and suggest that the hAd5 vector could be used for the effective delivery of vaccines or proteins in livestock animals in the field.
Keyword
MeSH Terms
Figure
Reference
-
1). Graham FL. Adenoviruses as expression vectors and recombinant vaccines. Trends Biotechnol. 1990; 8:85–7.
Article2). Barouch DH, Nabel GJ. Adenovirus vector-based vaccines for human immunodeficiency virus type 1. Hum Gene Ther. 2005; 16:149–56.
Article3). Lubeck MD, Davis AR, Chengalvala M, Natuk RJ, Morin JE, Molnar-Kimber K, et al. Immunogenicity and efficacy testing in chimpanzees of an oral hepatitis B vaccine based on live recombinant adenovirus. Proc Natl Acad Sci U S A. 1989; 86:6763–7.
Article4). Casimiro DR, Chen L, Fu TM, Evans RK, Caulfield MJ, Davies ME, et al. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J Virol. 2003; 77:6305–13.5). Kobinger GP, Feldmann H, Zhi Y, Schumer G, Gao G, Feldmann F, et al. Chimpanzee adenovirus vaccine protects against Zaire Ebola virus. Virology. 2006; 346:394–401.
Article6). Papp Z, Babiuk LA, Baca-Estrada ME. The effect of pre-existing adenovirus-specific immunity on immune responses induced by recombinant adenovirus expressing glycoprotein D of bovine herpesvirus type 1. Vaccine. 1999; 17:933–43.
Article7). Croyle MA, Patel A, Tran KN, Gray M, Zhang Y, Strong JE, et al. Nasal delivery of an adenovirus-based vaccine bypasses pre-existing immunity to the vaccine carrier and improves the immune response in mice. PLoS One. 2008; 3:e3548.
Article8). Moffatt S, Hays J, HogenEsch H, Mittal SK. Circumvention of vector-specific neutralizing antibody response by alternating use of human and non-human adenoviruses: implications in gene therapy. Virology. 2000; 272:159–67.
Article9). Seregin SS, Amalfitano A. Overcoming pre-existing adenovirus immunity by genetic engineering of adenovirus-based vectors. Expert Opin Biol Ther. 2009; 9:1521–31.
Article10). Bangari DS, Shukla S, Mittal SK. Comparative transduction efficiencies of human and nonhuman adenoviral vectors in human, murine, bovine, and porcine cells in culture. Biochem Biophys Res Commun. 2005; 327:960–6.11). Braucher DR, Henningson JN, Loving CL, Vincent AL, Kim E, Steitz J, et al. Intranasal vaccination with replication-defective adenovirus type 5 encoding influenza virus hemagglutinin elicits protective immunity to homologous challenge and partial protection to heterologous challenge in pigs. Clin Vaccine Immunol. 2012; 19:1722–9.
Article12). Kim SM, Lee KN, Lee SJ, Ko YJ, Lee HS, Kweon CH, et al. Multiple shRNAs driven by U6 and CMV promoter enhances efficiency of antiviral effects against foot-and-mouth disease virus. Antiviral Res. 2010; 87:307–17.
Article13). Perez-Martin E, Weiss M, Diaz-San Segundo F, Pacheco JM, Arzt J, Grubman MJ, et al. Bovine type III interferon significantly delays and reduces the severity of foot-and-mouth disease in cattle. J Virol. 2012; 86:4477–87.
Article14). Prevec L, Schneider M, Rosenthal KL, Belbeck LW, Derbyshire JB, Graham FL. Use of human adenovirus-based vectors for antigen expression in animals. J Gen Virol. 1989; 70:429–34.
Article15). Sun Y, Li HY, Zhang XJ, Chang TM, He F, Wang XP, et al. Comparison of the protective efficacy of recombinant adenoviruses against classical swine fever. Immunol Lett. 2011; 135:43–9.
Article16). Toro H, van Ginkel FW, Tang DC, Schemera B, Rodning S, Newton J. Avian influenza vaccination in chickens and pigs with replication-competent adenovirus-free human recombinant adenovirus 5. Avian Dis. 2010; 54:224–31.
Article17). Gogev S, Lemaire M, Thiry E. Prevalence of antibodies to human adenovirus type 5 in Belgian cattle. Vet Rec. 2001; 148:752–4.18). Kim SM, Lee KN, Park JY, Ko YJ, Joo YS, Kim HS, et al. Therapeutic application of RNA interference against foot-and-mouth disease virus in vitro and in vivo. Antiviral Res. 2008; 80:178–84.19). Reed LJ, Muench H. A simple method of estimating fifty percent end points. Am J Hygiene. 1938; 27:493–7.20). Ersching J, Hernandez MI, Cezarotto FS, Ferreira JD, Martins AB, Switzer WM, et al. Neutralizing antibodies to human and simian adenoviruses in humans and New-World monkeys. Virology. 2010; 407:1–6.
Article21). Brockmeier SL, Lager KM, Grubman MJ, Brough DE, Ettyreddy D, Sacco RE, et al. Adenovirus-mediated expression of interferon-alpha delays viral replication and reduces disease signs in swine challenged with porcine reproductive and respiratory syndrome virus. Viral Immunol. 2009; 22:173–80.22). Sun Y, Yang Y, Zheng H, Xi D, Lin M, Zhang X, et al. Co-expression of Erns and E2 genes of classical swine fever virus by replication-defective recombinant adenovirus completely protects pigs against virulent challenge with classical swine fever virus. Res Vet Sci. 2013; 94:354–60.
Article23). Lehmkuhl HD, Cutlip RC, Brogden KA. Seroepidemiologic survey for adenovirus infection in lambs. Am J Vet Res. 1993; 54:1277–9.24). Yeşilbağ K, Güngör B. Antibody prevalence against respiratory viruses in sheep and goats in North-Western Turkey. Trop Anim Health Prod. 2009; 41:421–5.
Article25). Elazhary MA, Dea S, Mittal KR, Higgins R. Prevalence of Antibodies to Swine Influenza Virus, Porcine Adenovirus Type 4 and Haemophilus pleuropneumoniae in Quebec Pig Farms with Respiratory Problems. Can Vet J. 1985; 26:190–2.26). Harrach B, Benko M. Phylogenetic analysis of adenovirus sequences. Methods Mol Med. 2007; 131:299–334.
Article27). W Woods L. Adenoviral diseases. IKB Elizabeth S, Williams , editors. Infectious diseases in wild mammals. LA: Iowa State University Press;2001. p. 202–13.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adenovirus Vectors: Excellent Tools for Vaccine Development
- Adenovirus Vector-mediated Gene Transfer into Human Trabecular Cell
- Genotype analysis of Cryptosporidium spp. prevalent in a rural village in Hwasun-gun, Republic of Korea
- Stromelysin Gene Expression in Rat Eye Mediated by the Adenovirus Vector
- Molecular epidemiology of Toxoplasma gondii in cattle in Korea