Further Increase of Vancomycin-Resistant Enterococcus faecium, Amikacin- and Fluoroquinolone-Resistant Klebsiella pneumoniae, and Imipenem-Resistant Acinetobacter spp. in Korea: 2003 KONSAR Surveillance
- Affiliations
-
- 1Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea. whonetkor@yumc.yonsei.ac.kr
- 2Department of Laboratory Medicine, Busan Medical Center, Busan, Korea.
- 3Department of Laboratory Medicine, Kosin University Gospel Hospital, Busan, Korea.
- 4Department of Laboratory Medicine, Kwandong University Myongji Hospital, Kyunggi, Korea.
- 5Department of Laboratory Medicine, Chonnam University Medical School, Kwangju, Korea.
- 6Department of Laboratory Medicine, Dongguk University, Kyongju Hospital, Kyongju, Korea.
- KMID: 1715872
- DOI: http://doi.org/10.3349/ymj.2006.47.1.43
Abstract
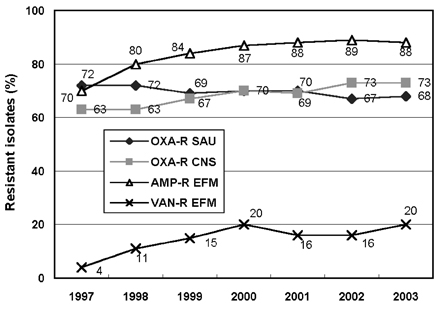
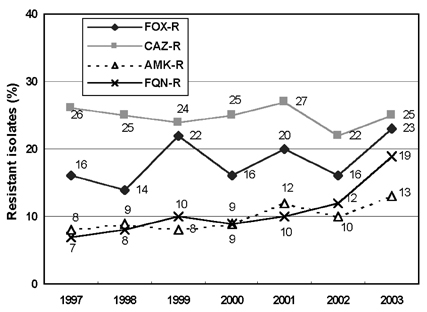
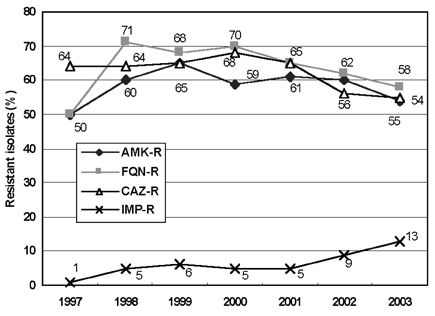
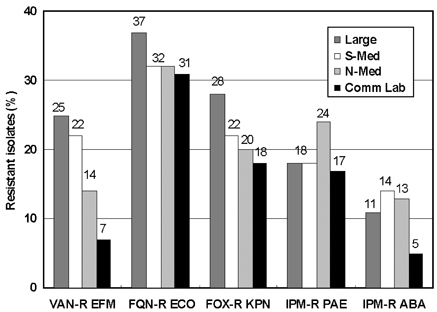
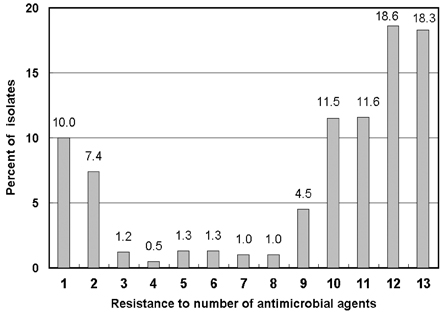
- Monitoring temporal trends of antimicrobial resistance can provide useful information for the empirical selection of antimicrobial agents to treat infected patients and for the control of nosocomial infections. In this study, we analyzed antimicrobial resistance of clinically relevant bacteria in 2003 at Korean hospitals and at a commercial laboratory. The following organism-antimicrobial agent resistance combinations were very prevalent: oxacillin-resistant Staphylococcus aureus (68%), expanded-spectrum cephalosporin-resistant Klebsiella pneumoniae (25%), and fluoroquinolone-resistant Escherichia coli (33%), Acinetobacter spp. (58%), and Pseudomonas aeruginosa (40%). Moreover, gradual increases in vancomycin-resistant Enterococcus faecium (20%), cefoxitin-resistant E. coli (10%) and K. pneumoniae (23%), and imipenem-resistant P. aeruginosa (20%) and Acinetobacter spp. (13%) were also observed. The resistance rates of Acinetobacter spp. to most antimicrobial agents at hospitals and at the commercial laboratory were similar. Among the Acinetobacter spp. isolated at a tertiary-care hospital, 46.2% were multidrug-resistant to 9-12 of 13 antimicrobial agents, and 18.3% were panresistant. The exclusion of duplicate isolates at a tertiary-care hospital significantly lowered the proportion of oxacillin-resistant S. aureus, vancomycin-resistant E. faecium, and fluoroquinolone-resistant E. coli.
Keyword
MeSH Terms
-
Vancomycin Resistance
Vancomycin/pharmacology
Population Surveillance
Microbial Sensitivity Tests
Korea/epidemiology
Klebsiella pneumoniae/drug effects/isolation & purification
Klebsiella Infections/drug therapy/epidemiology/microbiology
Imipenem/pharmacology
Humans
Gram-Positive Bacterial Infections/drug therapy/epidemiology/*microbiology
Gram-Negative Bacterial Infections/drug therapy/epidemiology/*microbiology
Gammaproteobacteria/*drug effects/isolation & purification
Fluoroquinolones/pharmacology
Enterococcus faecium/*drug effects/isolation & purification
*Drug Resistance, Bacterial
Anti-Bacterial Agents/*pharmacology
Amikacin/pharmacology
Acinetobacter Infections/drug therapy/epidemiology/microbiology
Acinetobacter/drug effects/isolation & purification
Figure
Cited by 6 articles
-
Multidrug-resistant Organisms and Healthcare-associated Infections
Mi-Na Kim
Hanyang Med Rev. 2011;31(3):141-152. doi: 10.7599/hmr.2011.31.3.141.Occurrence of a PCR-Positive but Culture-Negative Case for vanB Vancomycin-Resistant Enterococci in Stool Surveillance
Dahae Won, Ki Ho Hong, Kyungah Yun, Heungsup Sung, Mi-Na Kim
Lab Med Online. 2013;3(4):264-268. doi: 10.3343/lmo.2013.3.4.264.Molecular and Phenotypic Characteristics of 16S rRNA Methylase-producing Gram-negative Bacilli
Hyukmin Lee, Eun-Mi Koh, Chang Ki Kim, Jong Hwa Yum, Kyungwon Lee, Yunsop Chong
Korean J Clin Microbiol. 2010;13(1):19-26. doi: 10.5145/KJCM.2010.13.1.19.Antimicrobial Resistance of Enterococcal Isolates from Blood and Risk Factors for Vancomycin Resistant Enterococcal Bacteremia in a Tertiary Care University Hospital from 2003 to 2007
Kyung Sun Park, Myeong Hee Kim, Tae Sung Park, Jin Tae Suh, Hee Joo Lee
Korean J Clin Microbiol. 2010;13(2):59-67. doi: 10.5145/KJCM.2010.13.2.59.Chromosomal Mutations in
oprD, gyrA , andparC in Carbapenem ResistantPseudomonas aeruginosa
Ji Youn Sung, Hye Hyun Cho, Kye Chul Kwon, Sun Hoe Koo
Korean J Clin Microbiol. 2011;14(4):131-137. doi: 10.5145/KJCM.2011.14.4.131.Risk Factors for Prolonged Carriage and Reacquisition of Vancomycin-resistant Enterococci
Lee Dongsuk, Suk Park Eun, Yong Dongeun, Yong Choi Jun, Lee Kyungwon, Ha Jee Sun
Korean J Nosocomial Infect Control. 2013;20(1):19-28. doi: 10.14192/kjnic.2015.20.1.19.
Reference
-
1. Wise R. The relentless rise of resistance? J Antimicrob Chemother. 2004. 54:306–310.2. Bax R, Bywater R, Cornaglia G, Goosens H, Hunter P, Isham V, et al. Surveillance of antimicrobial resistancewhat, how and whither? Clin Microbiol Infect. 2001. 7:316–325.3. Morris AK, Masterton RG. Antibiotic resistance surveillance: action for international studies. J Antimicrob Chemother. 2002. 49:7–10.4. Felmingham D, Grueneberg RN. Alexander Project group. The Alexander project 1996-1997: latest susceptibility data from this international study of bacterial pathogens from community-acquired lower respiratory tract infections. J Antimicrob Chemother. 2000. 45:191–203.5. Van Beneden CA, Lexau C, Baughman W, Barnes B, Bennett N, Cassidy PM, et al. Aggregated antibiograms and monitoring of drug-resistant Streptococcus pneumoniae. Emerg Infect Dis. 2003. 9:1089–1095.6. Cornaglia G, Hryniewicz W, Jarlier V, Kahlmeter G, Mittermayer H, Stratchounski L, et al. European recommendations for antimicrobial resistance surveillance. Clin Microbiol Infect. 2004. 10:349–383.7. Chong Y, Lee K, Park YJ, Jeon DS, Lee MH, Kim MY, et al. Korean nationwide surveillance of antimicrobial resistance of bacteria in 1997. Yonsei Med J. 1998. 39:569–577.8. Lee K, Kim YA, Park YJ, Lee HS, Kim MY, Kim E-C, et al. Increasing prevalence of vancomyin-resistant enterococci, and cefoxitin-, imipenem- and fluoroquinolone-resistant gram-negative bacilli; a KONSAR study in 2002. Yonsei Med J. 2004. 45:598–608.9. Lee K, Lee WG, Uh Y, Ha GY, Cho J, Chong Y, et al. VIM- and IMP-type metallo-β-lactamase-producing Pseudomonas spp. and Acinetobacter spp. in Korean hospitals. Emerg Infect Dis. 2003. 9:868–871.10. Lee K, Lee M, Shin JH, Lee MH, Kang SH, Park AJ, et al. Plasmid mediated ampC genes in cefoxitin-resistant E. coli and K. pneumoniae clinical isolates. 2004. In : 44th Interscience Conference of Antimicrobial Agents and Chemotherapy; Washington, DC.. Abstr. C2-1339.11. Urban C, Segal-Maurer S, Rahal JJ. Considerations in control and treatment of nosocomial infections due to multidrug-resistant Acinetobacter baumannii. Clin Infect Dis. 2003. 36:1268–1274.12. National Committee for Clinical Laboratory Standards. Analysis and presentation of cumulative antimicrobial susceptibility test data. Approved guideline M39-A. 2002. Wayne, PA: NCCLS.13. Fridkin SK, Hill HA, Volova NV, Edwards JR, Lawton RM, Gaynes RP, et al. Intensive Care Antimicrobial Resistance Epidemiology (ICARE) Project Hospitals. Temporal changes in prevalence of antimicrobial resistance in 23 U.S. hospitals. Emerg Infect Dis. 2002. 8:697–701.14. Stelling JM, O'Brien TF. Surveillance of antimicrobial resistance: the WHONET program. Clin Infect Dis. 1997. 24:S157–S168.15. Sahm DF, Marsilio MK, Piazza G. Antimicrobial resistance in key bloodstream bacterial isolates: electronic surveillance with the Surveillance Network Database-USA. Clin Infect Dis. 1999. 29:259–263.16. National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing: thirteen's informational supplement. 2003. Wayne, PA: NCCLS.17. Pai H, Choi E-W, Lee H-J, Hong JY, Jacoby GA. Identification of CTX-M-14 extended-spectrum β-lactamase in clinical isolates of Shigella sonnei, Escherichia coli, and Klebsiella pneumoniae in Korea. J Clin Microbiol. 2001. 39:3747–3749.18. Bonnet R. Growing group of extended-spectrum β-lactamases: the CTX-M enzymes. Antimicrob Agents Chemother. 2004. 48:1–14.19. Yasunaka K, Kono K. Epidemiological study of methicillin-resistant Staphylococcus aureus at Fukuoka University Hospital. Microb Drug Resist. 1999. 5:207–213.20. Tiemersma EW, Bronzwaer SLAM, Lyytikainen O, Degener JE, Schrijnemakers P, Bruinsma N, et al. Methicillin-resistant Staphylococcus aureus in Europe, 1999-2002. Emerg Infect Dis. 2004. 10:1627–1634.21. Song J-H, Jung S-I, Ki HK, Shin M-H, Ko KS, Son JS, et al. Clinical outcomes of pneumococcal pneumonia caused by antibiotic-resistant strains in Asian countries: a study by the Asian Network for Surveillance of Resistant Pathogens. Clin Infect Dis. 2004. 38:1570–1578.22. Mera RM, Miller LA, Daniels JJD, Weil JG, White AR. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States over a 10-year period: Alexander Project. Diag Microbiol Infect Dis. 2005. 51:195–200.23. Treitman AN, Yarnold PR, Warren J, Noskin GA. Emerging incidence of Enterococcus faecium among hospital isolates (1993 to 2002). J Clin Microbiol. 2005. 43:462–463.24. Lauderdale TL, McDonald LC, Shiau YR, Chen PC, Wang HY, Lai JF, et al. The status of antimicrobial resistance in Taiwan among gram-negative pathogens: the Taiwan surveillance of antimicrobial resistance (TSAR) program, 2000. Diag Microbiol Infect Dis. 2004. 48:211–219.25. Hasegawa K, Chiba N, Kobayashi R, Murayama Y, Iwata S, Sunakawa K, et al. Rapidly increasing prevalence of β-lactamase-nonproducing, ampicillin-resistant Haemophilus influenzae type b in patients with meningitis. Antimicrob Agents Chemother. 2004. 48:1509–1514.26. Park M-S, Kang Y-H, Lee S-J, Song C-Y, Lee B-K. Characteristics of epidemic multidrug-resistant Salmonella enterica serovar Typhimurium DT104 strains first isolated in Korea. Korean J Infect Dis. 2002. 34:1–8.27. Jeong SH, Bae IK, Lee JH, Sohn SG, Kang GH, Jeon GJ, et al. Molecular characterization of extended-spectrum beta-lactamases produced by clinical isolates of Klebsiella pneumoniae and Escherichia coli from a Korean Nationwide Survey. J Clin Microbiol. 2004. 42:2902–2906.28. Livermore DM, Woodford N. Carbapenemases: a problem in waiting? Curr Opin Microbiol. 2000. 3:489–495.29. Kim IS, Oh WI, Song JH, Lee NY. Screening and identification of metallo-β-lactamase gene in clinical isolates of imipenem-resistant Pseudomonas aeruginosa. Korean J Lab Med. 2004. 24:177–182.30. Tsuji A, Kobayashi I, Oguri T, Inoue M, Yabuuchi E, Goto S. An epidemiological study of the susceptibility and frequency of multiple-drug-resistant strains of Pseudomonas aeruginosa isolated at medical institutes nationwide in Japan. J Infect Chemother. 2005. 11:64–70.31. Jeong SH, Lee K, Chong Y, Yum JH, Lee SH, Choi HJ, et al. Characterization of a new integron containing VIM-2, a metallo-β-lactamase gene cassette, in a clinical isolate of Enterobacter cloacae. J Antimicrob Chemother. 2003. 51:397–400.32. Hooper DC. The future of the quinolones. APUA Newsletter. 2001. 19:1–5.33. Abbo A, Navon-Venezia S, Hammer-Muntz O, Krichali T, Siegman-Igra Y, Carmeli Y. Multidrug-resistant Acinetobacter baumannii. Emerg Infect Dis. 2005. 11:22–29.34. Kuo LC, Teng LJ, Yu CJ, Ho SW, Hsueh PR. Dissemination of a clone of unusual phenotype of pandrug-resistant Acinetobacter baumannii at a university hospital in Taiwan. J Clin Microbiol. 2004. 42:1759–1763.35. Reis AO, Luz DAM, Tognim MCB, Sader HS, Gales AC. Polymyxin-resistant Acinetobacter spp. isolates; what is next? Emerg Infect Dis. 2003. 9:1025–1027.36. Critchley IA, Karlousky JA. Optimal use of antibiotic resistance surveillance systems. Clin Microbiol Infect. 2004. 10:502–511.37. Lee S-O, Cho YK, Kim S-Y, Lee ES, Park SY, Seo Y-H. Comparison of trends of resistance rates over 3 years calculated from results for all isolates and for the first isolate of a given species from a patient. J Clin Microbiol. 2004. 42:4776–4779.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Increase of Ceftazidime- and Fluoroquinolone-Resistant Klebsiella pneumoniae and Imipenem-Resistant Acinetobacter spp. in Korea: Analysis of KONSAR Study Data from 2005 and 2007
- Increasing Prevalence of Vancomycin-Resistant Enterococci, and Cefoxitin-, Imipenem- and Fluoroquinolone-Resistant Gram-Negative Bacilli: A KONSAR Study in 2002
- High Prevalence of Ceftazidime-Resistant Klebsiella pneumoniae and Increase of Imipenem-Resistant Pseudomonas aeruginosa and Acinetobacter spp. in Korea: a KONSAR Program in 2004
- Increase in the Prevalence of Carbapenem-Resistant Acinetobacter Isolates and Ampicillin-Resistant Non-Typhoidal Salmonella Species in Korea: A KONSAR Study Conducted in 2011
- Further Increases in Carbapenem-, Amikacin-, and Fluoroquinolone-Resistant Isolates of Acinetobacter spp. and P. aeruginosa in Korea: KONSAR Study 2009