Further Increases in Carbapenem-, Amikacin-, and Fluoroquinolone-Resistant Isolates of Acinetobacter spp. and P. aeruginosa in Korea: KONSAR Study 2009
- Affiliations
-
- 1Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea. whonetkor@yuhs.ac
- 2Department of Laboratory Medicine, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- 3Department of Laboratory Medicine, Hallym University College of Medicine, Seoul, Korea.
- 4Department of Laboratory Medicine, Seoul Clinical Laboratories, Seoul, Korea.
- 5Department of Laboratory Medicine, Hanyang University College of Medicine, Seoul, Korea.
- 6Department of Laboratory Medicine, Chonnam National University Medical School, Gwangju, Korea.
- 7Department of Laboratory Medicine, School of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 1108073
- DOI: http://doi.org/10.3349/ymj.2011.52.5.793
Abstract
- PURPOSE
The increasing prevalence of antimicrobial resistant bacteria has become a serious worldwide problem. The aim of this study was to analyze antimicrobial resistance data generated in 2009 by hospitals and commercial laboratories participating in the Korean Nationwide Surveillance of Antimicrobial Resistance program.
MATERIALS AND METHODS
Susceptibility data were collected from 24 hospitals and two commercial laboratories. In the analysis, resistance did not include intermediate susceptibility. Duplicate isolates were excluded from the analysis of hospital isolates, but not from the commercial laboratory isolates.
RESULTS
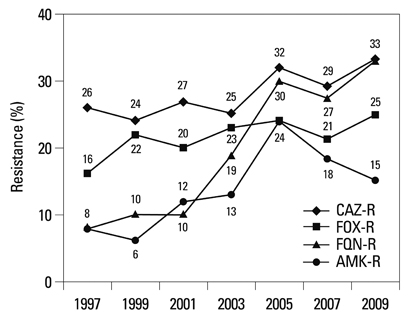
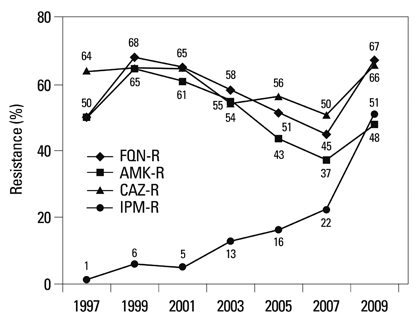
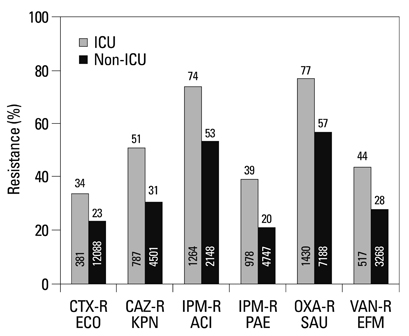
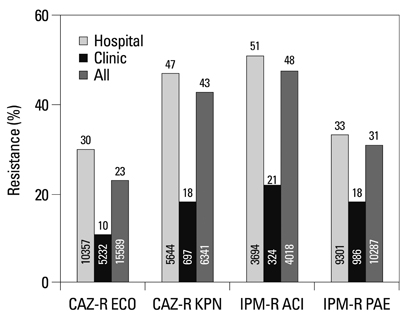
Among the hospital isolates, methicillin-resistant Staphylococcus aureus, penicillin G-non-susceptible Streptococcus pneumoniae based on meningitis breakpoint, and ampicillin-resistant Enterococcus faecium remained highly prevalent. The proportion of vancomycin-resistant E. faecium gradually increased to 29%. Ceftazidime-resistant Escherichia coli and Klebsiella pneumoniae increased to 17% and 33%, respectively, and fluoroquinolone-resistant K. pneumoniae, Acinetobacter spp. and Pseudomonas aeruginosa increased to 33%, 67% and 39%, respectively. Amikacin-resistant Acinetobacter spp. increased to 48%. Imipenem-resistant Acinetobacter spp. and P. aeruginosa increased to 51% and 26%, respectively. Higher resistance rates were observed in intensive care unit (ICU) isolates than in non-ICU isolates among the isolates from hospitals. Resistance rates were higher in hospital isolates than in clinic isolates among the isolates from commercial laboratories.
CONCLUSION
Among the hospital isolates, ceftazidime-resistant K. pneumoniae and fluoroquinolone-resistant K. pneumoniae, Acinetobacter spp., and P. aeruginosa further increased. The increase in imipenem resistance was slight in P. aeruginosa, but drastic in Acinetobacter spp. The problematic antimicrobial-organism combinations were much more prevalent among ICU isolates.
Keyword
MeSH Terms
-
Acinetobacter/*drug effects/isolation & purification
Acinetobacter Infections/drug therapy/microbiology
Amikacin/pharmacology
Anti-Bacterial Agents/pharmacology
Carbapenems/pharmacology
Cross Infection/drug therapy/microbiology
*Drug Resistance, Bacterial
Fluoroquinolones/pharmacology
Humans
Pseudomonas Infections/drug therapy/microbiology
Pseudomonas aeruginosa/*drug effects/isolation & purification
Republic of Korea
Figure
Cited by 11 articles
-
Multidrug-Resistant Acinetobacter spp.: Increasingly Problematic Nosocomial Pathogens
Kyungwon Lee, Dongeun Yong, Seok Hoon Jeong, Yunsop Chong
Yonsei Med J. 2011;52(6):879-891. doi: 10.3349/ymj.2011.52.6.879.Clinical Outcomes of Tigecycline in the Treatment of Multidrug-Resistant Acinetobacter baumannii Infection
Jung Ar Shin, Yoon Soo Chang, Hyung Jung Kim, Se Kyu Kim, Joon Chang, Chul Min Ahn, Min Kwang Byun
Yonsei Med J. 2012;53(5):974-984. doi: 10.3349/ymj.2012.53.5.974.Antimicrobial Resistance and Clones of Acinetobacter Species and Pseudomonas aeruginosa
Ji-Young Lee, Kwan Soo Ko
J Bacteriol Virol. 2012;42(1):1-8. doi: 10.4167/jbv.2012.42.1.1.Trend of Bacterial Resistance for the Past 50 Years in Korea and Future Perspectives - Gram-negative Bacteria
Kyungwon Lee
Infect Chemother. 2011;43(6):458-467. doi: 10.3947/ic.2011.43.6.458.Antimicrobial Resistance in Gram-positive Cocci: Past 50 Years, Present and Future
Jae-Hoon Song
Infect Chemother. 2011;43(6):443-449. doi: 10.3947/ic.2011.43.6.443.National Campaign for Appropriate Antibiotic Use in Korea
Doo Ryeon Chung, Jae-Hoon Song,
Infect Chemother. 2012;44(3):164-167. doi: 10.3947/ic.2012.44.3.164.Increase in the Prevalence of Carbapenem-Resistant Acinetobacter Isolates and Ampicillin-Resistant Non-Typhoidal Salmonella Species in Korea: A KONSAR Study Conducted in 2011
Dongeun Yong, Hee Bong Shin, Yong-kyun Kim, Jihyun Cho, Wee Gyo Lee, Gyoung Yim Ha, Tae Yeal Choi, Seok Hoon Jeong, Kyungwon Lee, Yunsop Chong,
Infect Chemother. 2014;46(2):84-93. doi: 10.3947/ic.2014.46.2.84.A Case of Community-Acquired Pneumonia Caused by Multidrug-Resistant Acinetobacter baumannii in Korea
Young Woong Son, In Young Jung, Mi Young Ahn, Yong Duk Jeon, Hea Won Ann, Jin Young Ahn, Nam Su Ku, Sang Hoon Han, Jun Young Choi, Young Goo Song, June Myung Kim
Infect Chemother. 2017;49(4):297-300. doi: 10.3947/ic.2017.49.4.297.Vancomycin-resistant Enterococcus avium Isolated from the Wound of a Patient with Diabetes Mellitus
Young Jin Ko, Hee Sook Shim, Hee-Won Moon, Mina Hur, Yeo-Min Yun
Lab Med Online. 2013;3(2):115-118. doi: 10.3343/lmo.2013.3.2.115.Antimicrobial Resistance in Asia: Current Epidemiology and Clinical Implications
Cheol-In Kang, Jae-Hoon Song
Infect Chemother. 2013;45(1):22-31. doi: 10.3947/ic.2013.45.1.22.Recent Trends in Antimicrobial Resistance in Intensive Care Units in Korea
Lee Yangsoon, Ah Kim Young, Song Wonkeun, Lee Hyukmin, Soo Lee Hye, Jin Jang Sook, Hoon Jeong Seok, Geun Hong Seong, Yong Dongeun, Lee Kyungwon, Chong Yunsop
Korean J Nosocomial Infect Control. 2013;19(1):29-36. doi: 10.14192/kjnic.2014.19.1.29.
Reference
-
1. Hsueh PR, Badal RE, Hawser SP, Hoban DJ, Bouchillon SK, Ni Y, et al. Epidemiology and antimicrobial susceptibility profiles of aerobic and facultative Gram-negative bacilli isolated from patients with intra-abdominal infections in the Asia-Pacific region: 2008 results from SMART (Study for Monitoring Antimicrobial Resistance Trends). Int J Antimicrob Agents. 2010. 36:408–414.
Article2. Samuelsen O, Toleman MA, Sundsfjord A, Rydberg J, Leegaard TM, Walder M, et al. Molecular epidemiology of metallo-β-lactamase-producing Pseudomonas aeruginosa isolates from Norway and Sweden shows import of international clones and local clonal expansion. Antimicrob Agents Chemother. 2010. 54:346–352.
Article3. Leclercq R, Derlot E, Duval J, Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988. 319:157–161.
Article4. Uttley AH, Collins CH, Naidoo J, George RC. Vancomycin-resistant enterococci. Lancet. 1988. 1:57–58.
Article5. Périchon B, Courvalin P. VanA-type vancomycin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2009. 53:4580–4587.6. Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD, et al. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2001. 45:1151–1161.
Article7. Walsh TR, Toleman MA, Poirel L, Nordmann P. Metallo-β-lactamases: the quiet before the storm? Clin Microbiol Rev. 2005. 18:306–325.
Article8. Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, et al. Characterization of a new metallo-β-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009. 53:5046–5054.
Article9. Morris AK, Masterton RG. Antibiotic resistance surveillance: action for international studies. J Antimicrob Chemother. 2002. 49:7–10.
Article10. Chong Y, Lee K, Park YJ, Jeon DS, Lee MH, Kim MY, et al. Korean Nationwide Surveillance of Antimicrobial Resistance of bacteria in 1997. Yonsei Med J. 1998. 39:569–577.
Article11. Lee K, Lee MA, Lee CH, Lee J, Roh KH, Kim S, et al. Increase of ceftazidime- and fluoroquinolone-resistant Klebsiella pneumoniae and imipenem-resistant Acinetobacter spp. in Korea: analysis of KONSAR study data from 2005 and 2007. Yonsei Med J. 2010. 51:901–911.
Article12. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; nineteenth informational supplement, M100-S19. 2009. Wayne, PA: CLSI.13. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-first informational supplement, M100-S21. 2011. Wayne, PA: CLSI.14. O'Brien TF, Stelling JM. WHONET: removing obstacles to the full use of information about antimicrobial resistance. Diagn Microbiol Infect Dis. 1996. 25:162–168.15. Fridkin SK, Hill HA, Volkova NV, Edwards JR, Lawton RM, Gaynes RP, et al. Temporal changes in prevalence of antimicrobial resistance in 23 US hospitals. Emerg Infect Dis. 2002. 8:697–701.
Article16. Van Beneden CA, Lexau C, Baughman W, Barnes B, Bennett N, Cassidy PM, et al. Aggregated antibiograms and monitoring of drug-resistant Streptococcus pneumoniae. Emerg Infect Dis. 2003. 9:1089–1095.
Article17. Sahm DF, Marsilio MK, Piazza G. Antimicrobial resistance in key bloodstream bacterial isolates: electronic surveillance with the Surveillance Network Database-USA. Clin Infect Dis. 1999. 29:259–263.
Article18. Felmingham D, Grüneberg RN. The Alexander Project 1996-1997: latest susceptibility data from this international study of bacterial pathogens from community-acquired lower respiratory tract infections. J Antimicrob Chemother. 2000. 45:191–203.
Article19. Giske CG, Cornaglia G. ESCMID Study Group on Antimicrobial Resistance Surveillance (ESGARS). Supranational surveillance of antimicrobial resistance: the legacy of the last decade and proposals for the future. Drug Resist Updat. 2010. 13:93–98.
Article20. Clinical and Laboratory Standards Institute. Analysis and presentation of cumulative antimicrobial susceptibility test data; Approved guideline-Third Edition. M39-A3. 2008. Wayne, PA: CLSI.21. Halstead DC, Gomez N, McCarter YS. Reality of developing a community-wide antibiogram. J Clin Microbiol. 2004. 42:1–6.
Article22. Jones RN. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis. 2010. 51:Suppl 1. S81–S87.
Article23. Llata E, Gaynes RP, Fridkin S. Measuring the scope and magnitude of hospital-associated infection in the United States: the value of prevalence surveys. Clin Infect Dis. 2009. 48:1434–1440.
Article24. Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med. 2010. 362:1804–1813.
Article25. Rosenthal VD, Maki DG, Jamulitrat S, Medeiros EA, Todi SK, Gomez DY, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary for 2003-2008, issued June 2009. Am J Infect Control. 2010. 38:95.e2–104.e2.
Article26. Zhao WH, Hu ZQ. β-lactamases identified in clinical isolates of Pseudomonas aeruginosa. Crit Rev Microbiol. 2010. 36:245–258.27. Lee K, Kim MN, Choi TY, Cho SE, Lee S, Whang DH, et al. Wide dissemination of OXA-type carbapenemases in clinical Acinetobacter spp. isolates from South Korea. Int J Antimicrob Agents. 2009. 33:520–524.
Article28. Falagas ME, Koletsi PK, Bliziotis IA. The diversity of definitions of multidrug-resistant (MDR) and pandrug-resistant (PDR) Acinetobacter baumannii and Pseudomonas aeruginosa. J Med Microbiol. 2006. 55:1619–1629.
Article29. Kallen AJ, Srinivasan A. Current epidemiology of multidrug resistant gram negative bacilli in the United States. Infect Control Hosp Epidemiol. 2010. 31:Suppl 1. S51–S54.30. Ko KS, Suh JY, Kwon KT, Jung SI, Park KH, Kang CI, et al. High rates of resistance to colistin and polymyxin B in subgroups of Acinetobacter baumannii isolates from Korea. J Antimicrob Chemother. 2007. 60:1163–1167.
Article31. Bae S, Lee J, Lee J, Kim E, Lee S, Yu J, et al. Antimicrobial resistance in Haemophilus influenzae respiratory tract isolates in Korea: results of a nationwide acute respiratory infections surveillance. Antimicrob Agents Chemother. 2010. 54:65–71.
Article32. Ramsey AM, Zilberberg MD. Secular trends of hospitalization with vancomycin-resistant Enterococcus infection in the United States, 2000-2006. Infect Control Hosp Epidemiol. 2009. 30:184–186.
Article33. Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect Control Hosp Epidemiol. 2008. 29:996–1011.
Article34. Jones RN, Sader HS, Moet GJ, Farrell DJ. Declining antimicrobial susceptibility of Streptococcus pneumoniae in the United States: report from the SENTRY Antimicrobial Surveillance Program (1998-2009). Diagn Microbiol Infect Dis. 2010. 68:334–336.
Article35. Watanabe M, Iyobe S, Inoue M, Mitsuhashi S. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991. 35:147–151.
Article36. Salabi AE, Toleman MA, Weeks J, Bruderer T, Frei R, Walsh TR. First report of the metallo-β-lactamase SPM-1 in Europe. Antimicrob Agents Chemother. 2010. 54:582.
Article37. Rhee JY, Park YK, Shin JY, Choi JY, Lee MY, Peck KR, et al. KPC-producing extreme drug-resistant Klebsiella pneumoniae isolate from a patient with diabetes mellitus and chronic renal failure on hemodialysis in South Korea. Antimicrob Agents Chemother. 2010. 54:2278–2279.
Article38. Dumartin C, L'Hériteau F, Péfau M, Bertrand X, Jarno P, Boussat S, et al. Antibiotic use in 530 French hospitals: results from a surveillance network at hospital and ward levels in 2007. J Antimicrob Chemother. 2010. 65:2028–2036.
Article39. Sykes R. The 2009 Garrod lecture: the evolution of antimicrobial resistance: a Darwinian perspective. J Antimicrob Chemother. 2010. 65:1842–1852.
Article40. Woodford N, Livermore DM. Infections caused by Gram-positive bacteria: a review of the global challenge. J Infect. 2009. 59:Suppl 1. S4–S16.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Increase in the Prevalence of Carbapenem-Resistant Acinetobacter Isolates and Ampicillin-Resistant Non-Typhoidal Salmonella Species in Korea: A KONSAR Study Conducted in 2011
- Antimicrobial Resistance and Clones of Acinetobacter Species and Pseudomonas aeruginosa
- Increase of Ceftazidime- and Fluoroquinolone-Resistant Klebsiella pneumoniae and Imipenem-Resistant Acinetobacter spp. in Korea: Analysis of KONSAR Study Data from 2005 and 2007
- Further Increase of Vancomycin-Resistant Enterococcus faecium, Amikacin- and Fluoroquinolone-Resistant Klebsiella pneumoniae, and Imipenem-Resistant Acinetobacter spp. in Korea: 2003 KONSAR Surveillance
- Multidrug-Resistant Acinetobacter spp.: Increasingly Problematic Nosocomial Pathogens