Increase of Ceftazidime- and Fluoroquinolone-Resistant Klebsiella pneumoniae and Imipenem-Resistant Acinetobacter spp. in Korea: Analysis of KONSAR Study Data from 2005 and 2007
- Affiliations
-
- 1Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea. whonetkor@yuhs.ac
- 2Department of Laboratory Medicine, Ewha Womans University Mokdong Hospital, Seoul, Korea.
- 3Department of Laboratory Medicine, Yeungnam University Hospital, Daegu, Korea.
- 4Department of Laboratory Medicine, Konyang University Hospital, Daejeon, Korea.
- 5Department of Laboratory Medicine, Gyeongsang National University Hospital, Jinju, Korea.
- 6Department of Laboratory Medicine, Inha University Hospital, Incheon, Korea.
- KMID: 1779638
- DOI: http://doi.org/10.3349/ymj.2010.51.6.901
Abstract
- PURPOSE
Antimicrobial resistance monitoring could be a useful source of information for treating and controlling nosocomial infections. We analyzed antimicrobial resistance data generated by Korean Hospitals and by a commercial laboratory in 2005 and 2007.
MATERIALS AND METHODS
Susceptibility data for 2005 and 2007 were collected from 37 and 41 hospitals, respectively, and from one commercial laboratory. Intermediate susceptibility was not included in the calculation of resistance rates.
RESULTS
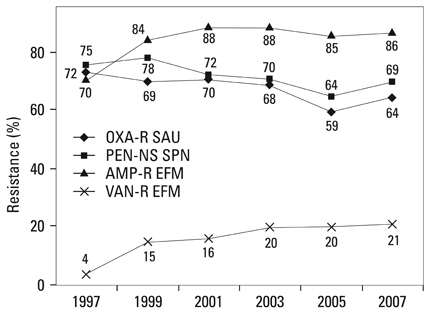
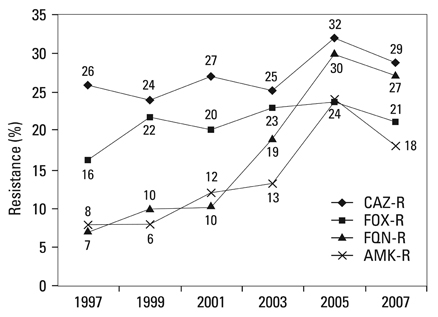
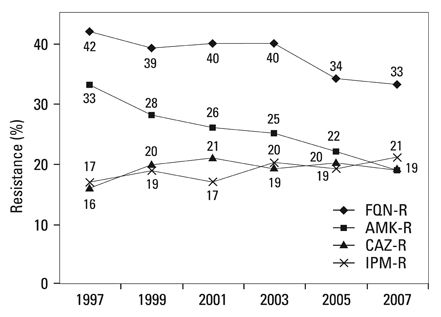
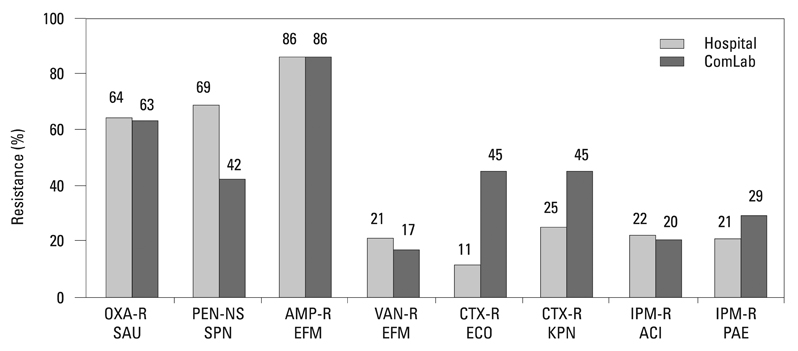
Methicillin-resistant Staphylococcus aureus (MRSA) (64%), third-generation cephalosporin-resistant Klebsiella pneumoniae (29%), fluoroquinolone-resistant Escherichia coli (27%), Pseudomonas aeruginosa (33%), and Acinetobacter spp. (48%), and amikacin-resistant P. aeruginosa (19%) and Acinetobacter spp. (37%) were prevalent in hospitals in 2007. A gradual increase of vancomycin-resistant Enterococcus faecium and imipenem-resistant Acinetobacter spp. was observed. Higher incidences of third-generation cephalosporin-resistant E. coli and K. pneumoniae and imipenem-resistant P. aeruginosa were found in the commercial laboratory than in the hospitals.
CONCLUSION
Methicillin-resistant S. aureus, third-generation cephalosporin-resistant K. pneumoniae, and fluoroquinolone-resistant E. coli, P. aeruginosa and Acinetobacter spp. remain prevalent in Korea, while the incidence of vancomycin-resistant E. faecium and imipenem-resistant Acinetobacter spp. has increased gradually. The higher prevalences of third-generation cephalosporin-resistant E. coli and K. pneumoniae, and imipenem-resistant P. aeruginosa in the commercial laboratory are a new concern.
Keyword
MeSH Terms
-
Acinetobacter/*metabolism
Bacterial Infections/drug therapy/*epidemiology
Ceftazidime/*pharmacology
Cross Infection/drug therapy/*epidemiology
*Drug Resistance, Bacterial
Escherichia coli/metabolism
Fluoroquinolones/*pharmacology
Humans
Imipenem/*pharmacology
Klebsiella Infections/*drug therapy
Klebsiella pneumoniae/*metabolism
Methicillin-Resistant Staphylococcus aureus/metabolism
Pseudomonas aeruginosa/metabolism
Republic of Korea
Vancomycin/pharmacology
Figure
Cited by 8 articles
-
Further Increases in Carbapenem-, Amikacin-, and Fluoroquinolone-Resistant Isolates of Acinetobacter spp. and P. aeruginosa in Korea: KONSAR Study 2009
Kyungwon Lee, Mi-Na Kim, Jae-Seok Kim, Hye Lim Hong, Jung Oak Kang, Jong Hee Shin, Yeon-Joon Park, Dongeun Yong, Seok Hoon Jeong, Yunsop Chong,
Yonsei Med J. 2011;52(5):793-802. doi: 10.3349/ymj.2011.52.5.793.Multidrug-Resistant Acinetobacter spp.: Increasingly Problematic Nosocomial Pathogens
Kyungwon Lee, Dongeun Yong, Seok Hoon Jeong, Yunsop Chong
Yonsei Med J. 2011;52(6):879-891. doi: 10.3349/ymj.2011.52.6.879.Profile of Antimicrobial Resistance of Staphylococcus aureus and Molecular Epidemiologic Characterization of Methicillin-resistant Staphylococcus aureus (MRSA) Isolated from Hands of People Using Multitude Facilities
Tae Sun Kim, Min Ji Kim, Sun Hee Kim, Hye-young Kee, Jin-jong Seo, Eun-Sun Kim, Yong-Un Moon, Puil Youl Ryu, Dong-Ryong Ha
Infect Chemother. 2012;44(4):289-298. doi: 10.3947/ic.2012.44.4.289.The Changing Patterns of Antibiotics Usage in Korea during 1981-2008
Youn Jeong Kim, Hyun Ji Chun, Jung Woo Lee, Kyung-Wook Hong, Sang Il Kim, Seong Heon Wie, Yang Ree Kim, Moon Won Kang
Infect Chemother. 2012;44(6):411-418. doi: 10.3947/ic.2012.44.6.411.The Efficacy and Safety of Arbekacin and Vancomycin for the Treatment in Skin and Soft Tissue MRSA Infection: Preliminary Study
Ji-Hee Hwang, Ju-Hyung Lee, Mi-Kyoung Moon, Ju-Sin Kim, Kyoung-Suk Won, Chang-Seop Lee
Infect Chemother. 2013;45(1):62-68. doi: 10.3947/ic.2013.45.1.62.Increase in the Prevalence of Carbapenem-Resistant Acinetobacter Isolates and Ampicillin-Resistant Non-Typhoidal Salmonella Species in Korea: A KONSAR Study Conducted in 2011
Dongeun Yong, Hee Bong Shin, Yong-kyun Kim, Jihyun Cho, Wee Gyo Lee, Gyoung Yim Ha, Tae Yeal Choi, Seok Hoon Jeong, Kyungwon Lee, Yunsop Chong,
Infect Chemother. 2014;46(2):84-93. doi: 10.3947/ic.2014.46.2.84.Therapeutic strategy for the management of multidrug-resistant gram-negative bacterial infections
Cheol-In Kang
J Korean Med Assoc. 2011;54(3):325-331. doi: 10.5124/jkma.2011.54.3.325.Multidrug-resistant Organisms and Healthcare-associated Infections
Mi-Na Kim
Hanyang Med Rev. 2011;31(3):141-152. doi: 10.7599/hmr.2011.31.3.141.
Reference
-
1. Jones RN, Masterton R. Determining the value of antimicrobial surveillance programs. Diagn Microbiol Infect Dis. 2001. 41:171–175.
Article2. Morris AK, Masterton RG. Antibiotic resistance surveillance: action for international studies. J Antimicrob Chemother. 2002. 49:7–10.
Article3. Cornaglia G, Hryniewicz W, Jarlier V, Kahlmeter G, Mittermayer H, Stratchounski L, et al. European recommendations for antimicrobial resistance surveillance. Clin Microbiol Infect. 2004. 10:349–383.
Article4. Llata E, Gaynes RP, Fridkin S. Measuring the scope and magnitude of hospital-associated infection in the United States: the value of prevalence surveys. Clin Infect Dis. 2009. 48:1434–1440.
Article5. Chong Y, Lee K, Park YJ, Jeon DS, Lee MH, Kim MY, et al. Korean Nationwide Surveillance of Antimicrobial Resistance of bacteria in 1997. Yonsei Med J. 1998. 39:569–577.
Article6. Lee K, Lim CH, Cho JH, Lee WG, Uh Y, Kim HJ, et al. High prevalence of ceftazidime-resistant Klebsiella pneumoniae and increase of imipenem-resistant Pseudomonas aeruginosa and Acinetobacter spp. in Korea: a KONSAR program in 2004. Yonsei Med J. 2006. 47:634–645.
Article7. Lee K, Lee WG, Uh Y, Ha GY, Cho J, Chong Y. Korean Nationwide Surveillance of Antimicrobial Resistance Group. VIM- and IMP-type metallo-beta-lactamase-producing Pseudomonas spp. and Acinetobacter spp. in Korean hospitals. Emerg Infect Dis. 2003. 9:868–871.
Article8. Lee K, Park AJ, Kim MY, Lee HJ, Cho JH, Kang JO, et al. Metallo-beta-lactamase-producing Pseudomonas spp. in Korea: high prevalence of isolates with VIM-2 type and emergence of isolates with IMP-1 type. Yonsei Med J. 2009. 50:335–339.
Article9. Lee K, Kim MN, Choi TY, Cho SE, Lee S, Whang DH, et al. Wide dissemination of OXA-type carbapenemases in clinical Acinetobacter spp. isolates from South Korea. Int J Antimicrob Agents. 2009. 33:520–524.10. Lee K, Lee M, Shin JH, Lee MH, Kang SH, Park AJ, et al. Prevalence of plasmid-mediated AmpC beta-lactamases in Escherichia coli and Klebsiella pneumoniae in Korea. Microb Drug Resist. 2006. 12:44–49.
Article11. Fridkin SK, Hill HA, Volova NV, Edwards JR, Lawton RM, Gaynes RP, et al. Temporal changes in prevalence of antimicrobial resistance in 23 US hospitals. Emerg Infect Dis. 2002. 8:697–701.12. Van Beneden CA, Lexau C, Baughman W, Barnes B, Bennett N, Cassidy PM, et al. Aggregated antibiograms and monitoring of drug-resistant Streptococcus pneumoniae. Emerg Infect Dis. 2003. 9:1089–1095.13. Bax R, Bywater R, Cornaglia G, Goosens H, Hunter P, Isham V, et al. Surveillance of antimicrobial resistance-what, how and whither? Clin Microbiol Infect. 2001. 7:316–325.14. Sahm DF, Marsilio MK, Piazza G. Antimicrobial resistance in key bloodstream bacterial isolates: electronic surveillance with the Surveillance Network Database-USA. Clin Infect Dis. 1999. 29:259–263.
Article15. Felmingham D, Grüeneberg RN. The Alexander project 1996-1997: latest susceptibility data from this international study of bacterial pathogens from community-acquired lower respiratory tract infections. J Antimicrob Chemother. 2000. 45:191–203.16. Jones ME, Draghi DC, Thornsberry C, Karlowsky JA, Sahm DF, Wenzel RP. Emerging resistance among bacterial pathogens in the intensive care unit - a European and North American Surveillance study (2000-2002). Ann Clin Microbiol Antimicrob. 2004. 3:14.17. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; nineteenth informational supplement, M100-S19. 2009. Wayne, PA: CLSI.18. Halstead DC, Gomez N, McCarter YS. Reality of developing a community-wide antibiogram. J Clin Microbiol. 2004. 42:1–6.
Article19. Lee SO, Cho YK, Kim SY, Lee ES, Park SY, Seo YH. Comparison of trends of resistance rates over 3 years calculated from results for all isolates and for the first isolate of a given species from a patient. J Clin Microbiol. 2004. 42:4776–4779.
Article20. Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect Control Hosp Epidemiol. 2008. 29:996–1011.
Article21. Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008. 21:538–582.
Article22. Niki Y, Hanaki H, Matsumoto T, Yagisawa M, Kohno S, Aoki N, et al. Nationwide surveillance of bacterial respiratory pathogens conducted by the Japanese Society of Chemotherapy in 2007: general view of the pathogens' antibacterial susceptibility. J Infect Chemother. 2009. 15:156–167.23. Styers D, Sheehan DJ, Hogan P, Sahm DF. Laboratory-based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Ann Clin Microbiol Antimicrob. 2006. 5:2.24. Weinstein MP, Klugman KP, Jones RN. Rationale for revised penicillin susceptibility breakpoints versus Streptococcus pneumoniae: coping with antimicrobial susceptibility in an era of resistance. Clin Infect Dis. 2009. 48:1596–1600.
Article25. Song JH, Jung SI, Ko KS, Kim NY, Son JS, Chang HH, et al. High prevalence of antimicrobial resistance among clinical Streptococcus pneumoniae isolates in Asia (an ANSORP study). Antimicrob Agents Chemother. 2004. 48:2101–2107.
Article26. Lee K, Park KH, Jeong SH, Lim HS, Shin JH, Yong D, et al. Further increase of vancomycin-resistant Enterococcus faecium, amikacin- and fluoroquinolone-resistant Klebsiella pneumoniae, and imipenem-resistant Acinetobacter spp. in Korea: 2003 KONSAR surveillance. Yonsei Med J. 2006. 47:43–54.27. Treitman AN, Yarnold PR, Warren J, Noskin GA. Emerging incidence of Enterococcus faecium among hospital isolates (1993 to 2002). J Clin Microbiol. 2005. 43:462–463.28. Roh KH, Uh Y, Kim JS, Kim HS, Shin DH, Song W. First outbreak of multidrug-resistant Klebsiella pneumoniae producing both SHV-12-type extended-spectrum beta-lactamase and DHA-1-type AmpC beta-lactamase at a Korean hospital. Yonsei Med J. 2008. 49:53–57.
Article29. Bell JM, Chitsaz M, Turnidge JD, Barton M, Walters LJ, Jones RN. Prevalence and significance of a negative extended-spectrum beta-lactamase (ESBL) confirmation test result after a positive ESBL screening test result for isolates of Escherichia coli and Klebsiella pneumoniae: results from the SENTRY Asia-Pacific Surveillance Program. J Clin Microbiol. 2007. 45:1478–1482.
Article30. Kitchel B, Sundin DR, Patel JB. Regional dissemination of KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2009. 53:4511–4513.31. Cai JC, Zhou HW, Zhang R, Chen GX. Emergence of Serratia marcescens, Klebsiella pneumoniae, and Escherichia coli isolates possessing the plasmid-mediated carbapenem-hydrolyzing beta-lactamase KPC-2 in intensive care units of a Chinese hospital. Antimicrob Agents Chemother. 2008. 52:2014–2018.
Article32. Hasegawa K, Chiba N, Kobayashi R, Murayama Y, Iwata S, Sunakawa K, et al. Rapidly increasing prevalence of beta-lactamase-nonproducing, ampicillin-resistant Haemophilus influenzae type b in patients with meningitis. Antimicrob Agents Chemother. 2004. 48:1509–1514.
Article33. Walkty A, DeCorby M, Nichol K, Karlowsky JA, Hoban DJ, Zhanel GG. In vitro activity of colistin (polymyxin E) against 3,480 isolates of gram-negative bacilli obtained from patients in Canadian hospitals in the CANWARD Study, 2007-2008. Antimicrob Agents Chemother. 2009. 53:4924–4926.
Article34. Park YK, Peck KR, Cheong HS, Chung DR, Song JH, Ko KS. Extreme drug resistance in Acinetobacter baumannii infections in intensive care units, South Korea. Emerg Infect Dis. 2009. 15:1325–1327.35. Yang HY, Lee HJ, Suh JT, Lee KM. Outbreaks of imipenem resistant Acinetobacter baumannii producing OXA-23 beta-lactamase in a tertiary care hospital in Korea. Yonsei Med J. 2009. 50:764–770.
Article36. Livermore DM, Woodford N. Carbapenemases: a problem in waiting? Curr Opin Microbiol. 2000. 3:489–495.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- High Prevalence of Ceftazidime-Resistant Klebsiella pneumoniae and Increase of Imipenem-Resistant Pseudomonas aeruginosa and Acinetobacter spp. in Korea: a KONSAR Program in 2004
- Increase in the Prevalence of Carbapenem-Resistant Acinetobacter Isolates and Ampicillin-Resistant Non-Typhoidal Salmonella Species in Korea: A KONSAR Study Conducted in 2011
- Increasing Prevalence of Vancomycin-Resistant Enterococci, and Cefoxitin-, Imipenem- and Fluoroquinolone-Resistant Gram-Negative Bacilli: A KONSAR Study in 2002
- Further Increases in Carbapenem-, Amikacin-, and Fluoroquinolone-Resistant Isolates of Acinetobacter spp. and P. aeruginosa in Korea: KONSAR Study 2009
- Further Increase of Vancomycin-Resistant Enterococcus faecium, Amikacin- and Fluoroquinolone-Resistant Klebsiella pneumoniae, and Imipenem-Resistant Acinetobacter spp. in Korea: 2003 KONSAR Surveillance