J Korean Med Sci.
2010 Jan;25(1):35-41. 10.3346/jkms.2010.25.1.35.
PPARgamma Agonist and Angiotensin II Receptor Antagonist Ameliorate Renal Tubulointerstitial Fibrosis
- Affiliations
-
- 1Department of Pathology, Inha University Hospital, Inha University Medical College, Incheon, Korea. jeeyhan@inha.ac.kr
- 2Division of Nephrology, Ansan Hospital, Korea University Medical College, Ansan, Korea.
- KMID: 1713829
- DOI: http://doi.org/10.3346/jkms.2010.25.1.35
Abstract
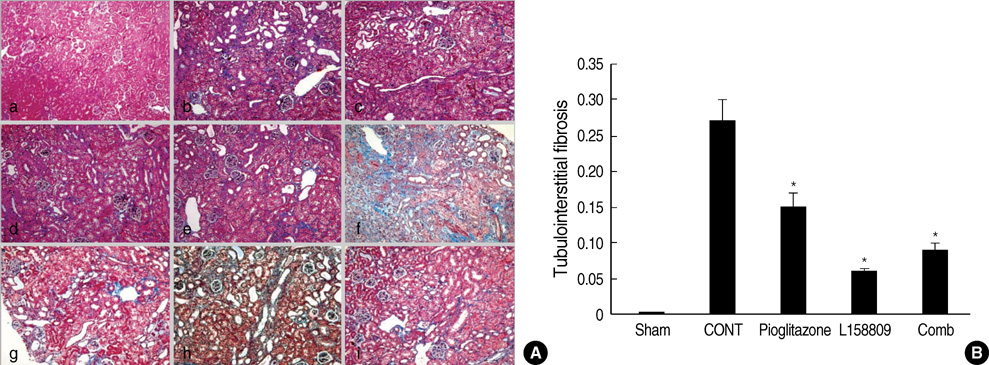
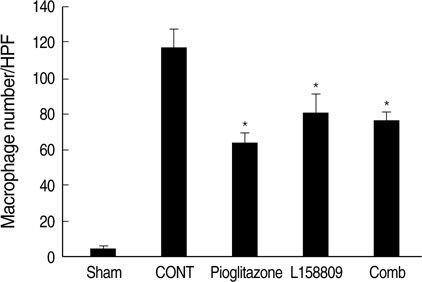
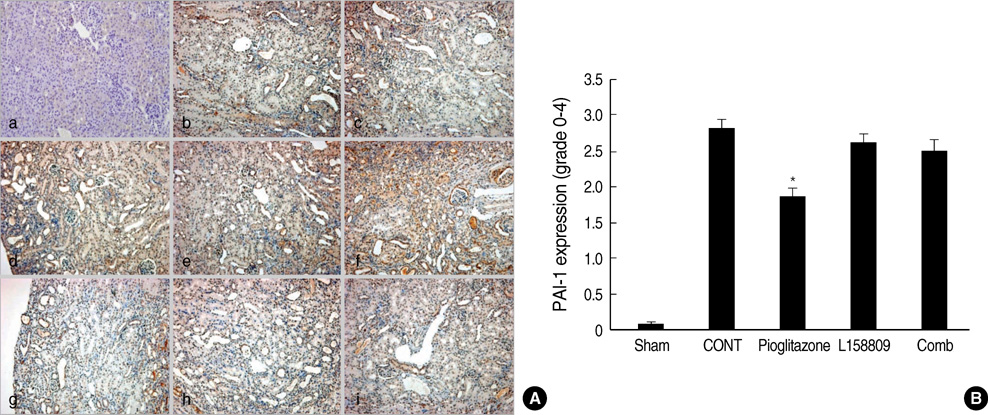
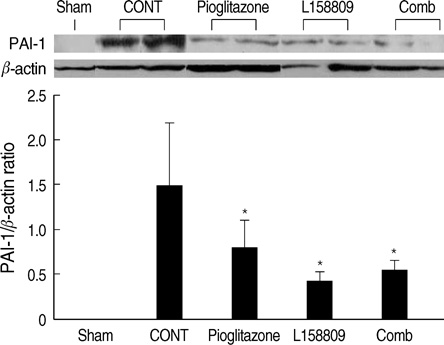
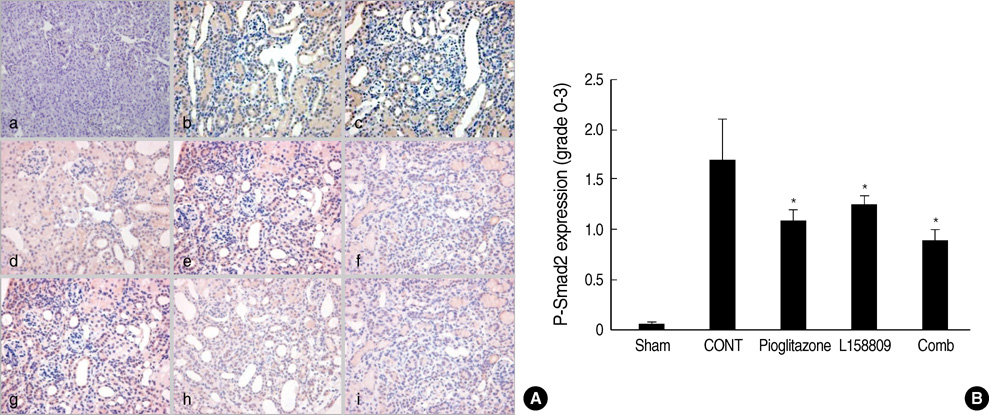
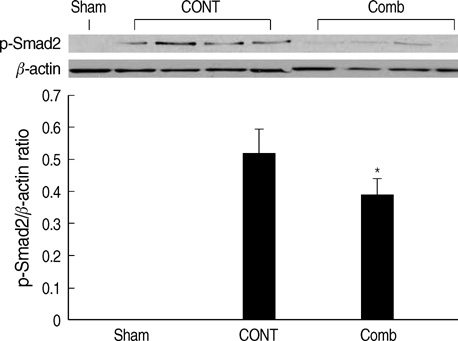
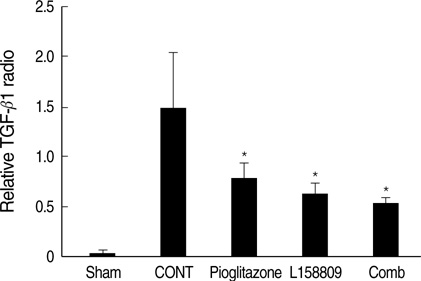
- The peroxisome proliferator activated receptor (PPAR)gamma agonist is used as antidiabetic agent with antihyperglycemic and antihyperinsulinemic actions. Beyond these actions, antifibrotic effects have been reported. We examined antifibrotic effects of PPARgamma agonist and interaction with angiotensin receptor antagonist in the unilateral ureteral obstruction (UUO) model. After UUO, mice were divided to four groups: no treatment (CONT), pioglitazone treatment, L158809 treatment, and L158809+ pioglitazone treatment. On day 14, CONT mice showed severe fibrosis and all treated mice showed decreased fibrosis. The immunohistochmistry of PAI-1, F4/80 and p-Smad2 demonstrated that their expressions were increased in CONT group and decreased in the all treated groups compared to CONT. PAI-1 and p-Smad2 determined from Western blotting, among treated groups, was decreased compared to CONT group. The expression of TGF-beta1 from real time RT PCR showed markedly increased in the CONT group and decreased in all treated groups compared to CONT. These data suggest the pioglitazone inhibited tubulointerstitial fibrosis, however, the synergism between pioglitazone and L158809 is not clear. Considering decreased expression of PAI-1 and TGF-beta/Smad2 in the treated groups, PAI-1 and TGF-beta are likely linked to the decreased renal tubulointerstitial fibrosis. According to these results, the PPARgamma agonist might be used in the treatment of renal fibrotic disease.
Keyword
MeSH Terms
-
*Angiotensin Receptor Antagonists
Animals
Antigens, Differentiation/metabolism
Disease Models, Animal
Fibrosis
Hypoglycemic Agents/pharmacology
Kidney/metabolism/*pathology
Male
Mice
Mice, Inbred C57BL
PPAR gamma/*agonists
Plasminogen Activator Inhibitor 1/metabolism
Smad2 Protein/metabolism
Thiazolidinediones/pharmacology
Transforming Growth Factor beta1/genetics/metabolism
Ureteral Obstruction/metabolism/pathology
Angiotensin Receptor Antagonists
Antigens, Differentiation
Hypoglycemic Agents
PPAR gamma
Plasminogen Activator Inhibitor 1
Smad2 Protein
Thiazolidinediones
Transforming Growth Factor beta1
Figure
Reference
-
1. Guan Y, Zhang Y, Breyer MD. The role of PPARs in the transcription control of the cellular processes. Drug News Perspect. 2002. 15:147–154.2. McCarthy KJ, Routh RE, Shaw W, Walsh K, Welbourne TC, Johnson JH. Troglitazone halts diabetic glomerulosclerosis by blockade of mesangial expansion. Kidney Int. 2000. 58:2341–2350.
Article3. Smith SA, Lister CA, Toseland CD, Buckingham RE. Rosiglitazone prevents the onset of hyperglycemia and proteinuria in the Zucker diabetic fatty rats. Diabetes Obes Metab. 2000. 2:363–372.4. Ma LJ, Marcantoni C, Linton MF, Fazio S, Fogo AB. Peroxisome proliferator-activated receptor-gamma agonist troglitazone protects against non-diabetic glomerulosclerosis in rats. Kidney Int. 2001. 59:1899–1910.5. Ghosh SS, Gehr TW, Ghosh S, Fakhry I, Sica DA, Lyall V, Schoolwerth AC. PPARgamma ligand attenuates PDGF-induced mesangial cell proliferation: role of MAP kinase. Kidney Int. 2003. 64:52–62.6. Routh RE, Johnson JH, McCarthy KJ. Troglitazone suppresses the secretion of type I collagen by mesangial cells in vitro. Kidney Int. 2002. 61:1365–1376.
Article7. Guo B, Koya D, Isono M, Sugimoto T, Kashiwagi A, Haneda M. Peroxisome proliferator-activated receptor-gamma ligands inhibit TGF-beta 1-induced fibronectin expression in glomerulomesangial cells. Diabetes. 2004. 53:200–208.8. Eddy AA. Experimental insights into the tubulointerstitial disease accompanying primary glomerular lesions. J Am Soc Nephrol. 1994. 5:1273–1287.
Article9. Miyajima A, Chen J, Lawrence C, Ledbetter S, Soslow RA, Stern J, Jha S, Pigato J, Lemer ML, Poppas DP, Vaughan ED, Felsen D. Antibody to transforming growth factor-beta ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int. 2000. 58:2301–2313.10. Duymelinck C, Dauwe SE, De Greef KE, Ysebaert DK, Verpooten GA, De Broe ME. TIMP-1 gene expression and PAI-1 antigen after unilateral ureteral obstruction in adult male rat. Kidney Int. 2000. 58:1186–1201.11. Ma J, Nishimura H, Fogo A, Kon V, Inagami T, Ichikawa I. Accelerated fibrosis and collagen deposition develop in the renal interstitium of angiotensin type 2 receptor null mutant mice during ureteral obstruction. Kidney Int. 1998. 53:937–944.12. Bonegio RG, Fuhro R, Wang Z, Valeri CR, Andry C, Salant DJ, Lieberthal W. Rapamycin ameliorates proteinuria associated tubulointerstitial fibrosis inflammation and fibrosis in experimental membranous nephropathy. J Am Soc Nephrol. 2005. 16:2063–2072.13. Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol. 2001. 159:1465–1475.
Article14. Buckinham RE, Al-Barazanji KA, Toseland CD, Slaughter M, Connor SC, West A, Bond B, Turner NC, Clapham JC. Peroxisom proliferator-activated receptor gamma agonist, rosiglitazone, protects against nephropathy and pancreatic islet abnormalities in Zucker fatty rats. Diabetes. 1998. 47:1326–1334.15. Fujii M, Takemura R, Yamaguchi M, Hasegawa G, Shigeta H, Nakano K, Kondo M. Troglitazone (CS-045) ameliorates albuminuria in streptozotocin-induced diabetic rats. Metabolism. 1997. 46:981–983.
Article16. Imano E, Kanda T, Nakatani Y, Nishida T, Arai K, Motomura M, Kajimoto Y, Yamasaki Y, Hori M. Effect of troglitazone halts diabetic glomerulosclerosis by blockade of mesangial expansion. Diabetes Care. 1998. 21:2135–2139.17. Zafirious S, Stanners SR, Saad S, Polhill TS, Poronnik P, Pollock CA. Pioglitazone inhibits cell growth and reduces matrix production in human kidney fibroblasts. J Am Soc Nephrol. 2005. 16:638–645.18. Eddy AA. Plasminogen activator inhibitor-1 and the kidney. Am J Physiol Renal Physiol. 2002. 283:F209–F220.
Article19. Fogo AB. The role of angiotensin II and plasminogen activator inhibitor-1 in progressive glomerulosclerosis. Am J Kidney Dis. 2000. 35:179–188.
Article20. Brown NJ, Vaughan DE, Fogo AB. Aldosterone and PAI-1: implications for renal injury. J Nephrol. 2002. 15:230–235.21. Panchapakesan U, Sumual S, Pollock CA, Chen X. PPARgamma agonists exert antifibrotic effects in renal tubular cells exposed to high glucose. Am J Physiol Renal Physiol. 2005. 289:F1153–F1158.22. Li Y, Wen X, Spataro BC, Hu K, Dai C, Liu Y. Hepatocyte growth factor is a downstream effector that mediates the antifibrotic action of peroxisome proliferator-activated receptor-gamma agonists. J Am Soc Nephrol. 2006. 17:54–65.23. Massague J, Chen YG. Controlling TGF-beta signaling. Genes Dev. 2000. 14:627–644.24. Eddy AA. Molecular basis of renal fibrosis. Pediatr Nephrol. 2000. 15:290–301.
Article25. Diamond JR. Macrophages and progressive renal disease in experimental hydronephrosis. Am J Kidney Dis. 1995. 26:133–140.
Article26. Ishidoya S, Morrissey J, McCracken R, Reyes A, Klahr S. Angiotensin II receptor antagonist ameliorates renal tubulointerstitial fibrosis caused by unilateral ureteral obstruction. Kidney Int. 1995. 47:1285–1294.
Article27. Yao Q, Ayala ER, Qian JQ, Stenvinkel P, Axelsson J, Lindholm B. A combination of PPAR gamma agonist and an angiotensin receptor blocker attenuates proinflammatory signaling and stimulates expression of Smad7 in human peritoneal mesothelial cells. Clin Nephrol. 2007. 68:295–301.28. Schmerbach K, Schefe JH, Krikov M, Müller S, Villringer A, Kintscher U, Unger T, Thoene-Reineke C. Comparison between single and combined treatment with candesartan and pioglitazone following transient focal ischemia rat brain. Brain Res. 2008. 7:225–233.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Renal Interstitial Fibrosis and Angiotensin Inhibition
- Angiotensin converting enqyme inhibitor and angiotensin II AT1 receptor antagonist in progressive renal disease
- Renal fibrosis
- Effect of Angiotensin II Receptor Blockers in Proteinuric IgA Nephropathy
- The Therapeutic Effect of Angiotensin II Receptor Antagonist in Idiopathic Pulmonary Fibrosis