J Korean Med Sci.
2008 Apr;23(2):262-269. 10.3346/jkms.2008.23.2.262.
Regulation of Inhibitors of Differentiation Family Proteins by Thyroid-Stimulating Hormone in FRTL-5 Thyroid Cells
- Affiliations
-
- 1Division of Endocrinology, Department of Internal Medicine, Eulji University School of Medicine, Daejeon, Korea.
- 2Laboratory of Endocrine Cell Biology, Division of Endocrinology, Department of Internal Medicine, Chungnam National University School of Medicine, Daejeon, Korea. minhos@cnu.ac.kr
- KMID: 1713459
- DOI: http://doi.org/10.3346/jkms.2008.23.2.262
Abstract
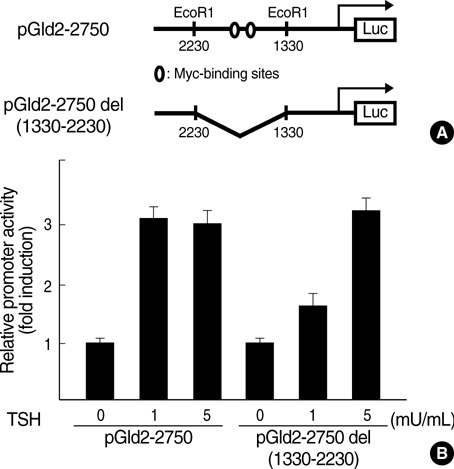
- Members of the inhibitors of differentiation (Id) family of helix-loop-helix (HLH) proteins are known to play important roles in the proliferation and differentiation of many cell types. Thyroid-stimulating hormone (TSH) regulates proliferation and differentiation by activating TSH receptor (TSHR) in thyrocytes. In this study, we found that Id2, one of the Id family proteins, is a major target for regulation by TSH in FRTL-5 thyroid cells. TSH rapidly increases the Id2 mRNA level in FRTL-5 thyroid cells but the Id2 protein showed biphasic regulatory patterns, being transiently reduced and subsequently induced by TSH treatment. Transient reduction of Id2 protein was noted within 2 hr of TSH treatment and was mediated by proteasomal degradation. Moreover, reduced Id2 expression correlated with the activity of the phosphatidylinositol 3 kinase pathway, which is activated by TSH. Although TSH increases the activity of the Id2 promoter, TSH-induced activation of this promoter was independent of c-Myc. Id2 did not alter TTF-1- and Pax-8-mediated effects on the regulation of the Tg promoter. Thus, in summary, we found that TSH regulates Id2 expression, but that Id2 does not alter the expression of thyroid-specific genes, such as Tg, in FRTL-5 thyroid cells.
Keyword
MeSH Terms
-
1-Phosphatidylinositol 3-Kinase/metabolism
Animals
Cattle
Cell Differentiation
Cell Proliferation
*Gene Expression Regulation
Inhibitor of Differentiation Protein 2/metabolism
Insulin/metabolism
Paired Box Transcription Factors/metabolism
Promoter Regions, Genetic
Proto-Oncogene Proteins c-myc/metabolism
Rats
Thyroglobulin/metabolism
Thyroid Gland/*cytology
Thyrotropin/*metabolism
Figure
Reference
-
1. Kimura T, Van Keymeulen A, Golstein J, Fusco A, Dumont JE, Roger PP. Regulation of thyroid cell proliferation by TSH and other factors: a critical evaluation of in vitro models. Endocr Rev. 2001. 22:631–656.
Article2. Cass LA, Meinkoth JL. Ras signaling through PI3K confers hormone-independent proliferation that is compatible with differentiation. Oncogene. 2000. 19:924–932.
Article3. Tsygankova OM, Saavedra A, Rebhun JF, Quilliam LA, Meinkoth JL. Coordinated regulation of Rap1 and thyroid differentiation by cyclic AMP and protein kinase A. Mol Cell Biol. 2001. 21:1921–1929.
Article4. Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002. 296:1655–1657.
Article5. Suh JM, Song JH, Kim DW, Kim H, Chung HK, Hwang JH, Kim JM, Hwang ES, Chung J, Han JH, Cho BY, Ro HK, Shong M. Regulation of the phosphatidylinositol 3-kinase, Akt/protein kinase B, FRAP/mammalian target of rapamycin, and ribosomal S6 kinase 1 signaling pathways by thyroid-stimulating hormone (TSH) and stimulating type TSH receptor antibodies in the thyroid gland. J Biol Chem. 2003. 278:21960–21971.
Article6. Francis-Lang H, Zannini M, De Felice M, Berlingieri MT, Fusco A, Di Lauro R. Multiple mechanisms of interference between transformation and differentiation in thyroid cells. Mol Cell Biol. 1992. 12:5793–5800.
Article7. Shimura H, Okajima F, Ikuyama S, Shimura Y, Kimura S, Saji M, Kohn LD. Thyroid-specific expression and cyclic adenosine 3'5'-monophosphate autoregulation of the thyrotropin receptor gene involves thyroid transcription factor-1. Mol Endocrinol. 1994. 8:1049–1069.
Article8. Kohn LD, Shimura H, Shimura Y, Hidaka A, Giuliani C, Napolitano G, Ohmori M, Laglia G, Saji M. The thyrotropin receptor. Vitam Horm. 1995. 50:287–384.
Article9. Damante G, Di Lauro R. Thyroid-specific gene expression. Biochim Biophys Acta. 1994. 1218:255–266.
Article10. Shimura Y, Shimura H, Ohmori M, Ikuyama S, Kohn LD. Identification of a novel insulin-responsive element in the rat thyrotropin receptor promoter. J Biol Chem. 1994. 269:31908–31914.
Article11. Santisteban P, Acebron A, Polycarpou-Schwarz M, Di Lauro R. Insulin and insulin-like growth factor I regulate a thyroid-specific nuclear protein that binds to the thyroglobulin promoter. Mol Endocrinol. 1992. 6:1310–1317.
Article12. Endo T, Kaneshige M, Nakazato M, Ohmori M, Harii N, Onaya T. Thyroid transcription factor-1 activates the promoter activity of rat thyroid Na+/I- symporter gene. Mol Endocrinol. 1997. 11:1747–1755.
Article13. Dohan O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M, Ginter CS, Carrasco N. The Sodium/Iodide Symporter (NIS): Characterization, regulation, and medical significance. Endocr Rev. 2003. 24:48–77.14. Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990. 61:49–59.
Article15. Deed RW, Jasiok M, Norton JD. Nucleotide sequence of the cDNA encoding human helix-loop-helix Id-1 protein: identification of functionally conserved residues common to Id proteins. Biochim Biophys Acta. 1994. 1219:160–162.
Article16. Yokota Y. Id and development. Oncogene. 2001. 20:8290–8298.
Article17. Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S, Gruss P. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999. 397:702–706.
Article18. Norton JD, Atherton GT. Coupling of cell growth control and apoptosis functions of Id proteins. Mol Cell Biol. 1998. 18:2371–2381.
Article19. Florio M, Hernandez MC, Yang H, Shu HK, Cleveland JL, Israel MA. Id2 promotes apoptosis by a novel mechanism independent of dimerization to basic helix-loop-helix factors. Mol Cell Biol. 1998. 18:5435–5444.
Article20. Iavarone A, Garg P, Lasorella A, Hsu J, Israel MA. The helix-loop-helix protein Id-2 enhances cell proliferation and binds to the retinoblastoma protein. Genes Dev. 1994. 8:1270–1284.
Article21. Lasorella A, Iavarone A, Israel MA. Id2 specifically alters regulation of the cell cycle by tumor suppressor proteins. Mol Cell Biol. 1996. 16:2570–2578.
Article22. Lasorella A, Noseda M, Beyna M, Yokota Y, Iavarone A. Id2 is a retinoblastoma protein target and mediates signaling by Myc oncoproteins. Nature. 2000. 407:592–598.23. Zebedee Z, Hara E. Id proteins in cell cycle control and cellular senescence. Oncogene. 2001. 20:8317–8325.
Article24. Roberts EC, Deed RW, Inoue T, Norton JD, Sharrocks AD. Id helix-loop-helix proteins antagonize pax transcription factor activity by inhibiting DNA binding. Mol Cell Biol. 2001. 21:524–533.
Article25. Simonson MS, Rooney A, Herman WH. Expression and differential regulation of Id1, a dominant negative regulator of basic helix-loop-helix transcription factors, in glomerular mesangial cells. Nucleic Acids Res. 1993. 21:5767–5774.
Article26. Kohn LD, Valente WA, Grollman EF, Aloj SM, Vitti P. Clinical determination and/or quantification of thyrotropin and a variety of thyroid stimulatory or inhibitory factors performed in vitro with an improved cell line. U S Patent. 1986. 4:609–622.27. Park ES, Kim H, Suh JM, Park SJ, Kwon OY, Kim YK, Ro HK, Cho BY, Chung J, Shong M. Thyrotropin induces SOCS-1 (suppressor of cytokine signaling-1) and SOCS-3 in FRTL-5 thyroid cells. Mol Endocrinol. 2000. 14:440–448.
Article28. Kim H, Suh JM, Hwang ES, Kim DW, Chung HK, Song JH, Hwang JH, Park KC, Ro HK, Jo EK, Chang JS, Lee TH, Lee MS, Kohn LD, Shong M. Thyrotropin-mediated repression of class II trans-activator expression in thyroid cells: involvement of STAT3 and suppressor of cytokine signaling. J Immunol. 2003. 171:616–627.
Article29. Bounpheng MA, Dimas JJ, Dodds SG, Christy BA. Degradation of Id proteins by the ubiquitin-proteasome pathway. FASEB J. 1999. 13:2257–2264.
Article30. Wojcik C. Inhibition of the proteasome as a therapeutic approach. Drug Discov Today. 1999. 4:188–189.31. Rui L, Fisher TL, Thomas J, White MF. Regulation of insulin/insulin-like growth factor-1 signaling by proteasome-mediated degradation of insulin receptor substrate-2. J Biol Chem. 2001. 276:40362–40367.
Article32. Bounpheng MA, Melnikova IN, Dimas JJ, Christy BA. Identification of a novel transcriptional activity of mammalian Id proteins. Nucleic Acids Res. 1999. 27:1740–1746.
Article33. Kaliman P, Viñals F, Testar X, Palacín M, Zorzano A. Phosphatidylinositol 3-kinase inhibitors block differentiation of skeletal muscle cells. J Biol Chem. 1996. 271:19146–19151.
Article34. Kimura K, Hattori S, Kabuyama Y, Shizawa Y, Takayanagi J, Nakamura S, Toki S, Matsuda Y, Onodera K, Fukui Y. Neurite outgrowth of PC12 cells is suppressed by wortmannin, a specific inhibitor of phosphatidylinositol 3-kinase. J Biol Chem. 1994. 269:18961–18967.
Article35. Sun XJ, Goldberg JL, Qiao LY, Mitchell JJ. Insulin-induced insulin receptor substrate-1 degradation is mediated by the proteasome degradation pathway. Diabetes. 1999. 48:1359–1364.
Article36. Lee AV, Gooch JL, Oesterreich S, Guler RL, Yee D. . Mol Cell Biol. 2000. 20:1489–1496.37. Dere WH, Hirayu H, Rapoport B. TSH and cAMP enhance expression of the myc proto-oncogene in cultured thyroid cells. Endocrinology. 1985. 117:2249–2251.38. Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, Gonzalez FJ. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996. 10:60–69.
Article39. Pasca di Magliano M, Di Lauro R, Zannini M. Pax8 has a key role in thyroid cell differentiation. Proc Natl Acad Sci USA. 2000. 97:13144–13149.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Difference of Thyroid Stimulating Antidody Activities Measured in Chinese Hamster Ovary Cells Stably Transfected with Human TSH Receptor and in FRTL-5 Cells in Graves Disease and Its Clinical Correlations
- Effect of ceramide on apoptosis and phospholipase D activity in FRTL-5 thyroid cells
- Assay of Thyrotropin Receptor Antibodies with Recombinant Human Thyrotropin Receptor Expressed on Chinese Hamster Ovary Cells
- Simultaneous measurement of thyroid growth stimulating antibody and thyroid adenylate cyclase stimulating antibody using FRTL-5 cells in patients with Graves' disease
- Effect of Lithium on Na+/I- Symporter Gene Expression in Rat Thyroid FRTL-5 Cells