J Korean Med Sci.
2007 Oct;22(5):855-861. 10.3346/jkms.2007.22.5.855.
Epstein-Barr Virus, Beta-Catenin, and E-cadherin in Gastric Carcinomas
- Affiliations
-
- 1Department of Surgery, Seoul National University Boramae Hospital, Seoul, Korea.
- 2Department of Pathology, Seoul National University Boramae Hospital, Seoul, Korea. meesoch@snu.ac.kr
- 3Department of Internal Medicine, Seoul National University Boramae Hospital, Seoul, Korea.
- 4Department of Surgery, Seoul National University College of Medicine, Seoul, Korea.
- 5Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
- 6Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1713293
- DOI: http://doi.org/10.3346/jkms.2007.22.5.855
Abstract
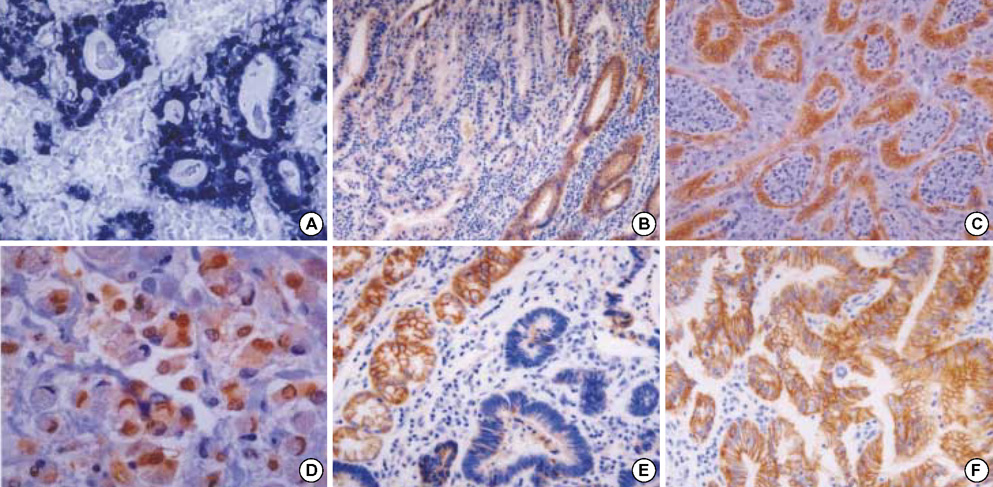
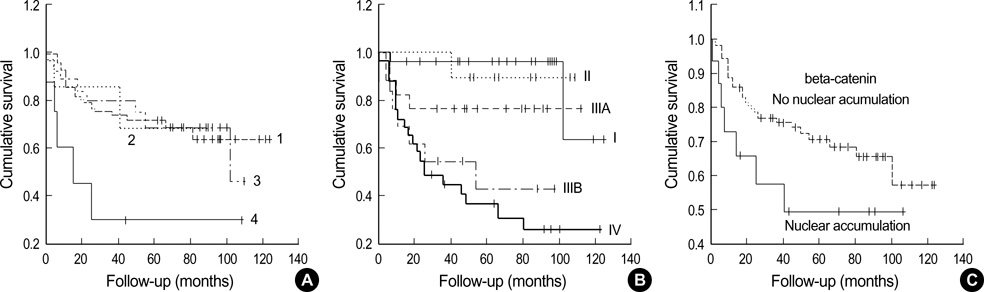
- Activated beta-catenin is suggested to inhibit NF-kappaB activation, and we previously demonstrated that NF-kappaB nuclear positivity was more frequent in Epstein-Barr virus (EBV)-infected gastric carcinomas. It is controversial that beta-catenin and E-cadherin are prognostic markers in gastric carcinomas. To define a relationship between beta-catenin and EBV, and the prognostic value of beta-catenin and E-cadherin, we analyzed in situ hybridization for EBV-encoded small RNAs, betacatenin, and E-cadherin immunohistochemistry, and clinicophatological features in 111 gastric carcinomas. EBV infection was detected in seven carcinomas (6.3%); none of seven showed beta-catenin nuclear accumulation, and five out of seven revealed beta-catenin membranous loss or cytoplamic expression. Eighty cases (72.1%) showed beta-catenin alteration; i.e., loss of membrane staining in 65 (58.6 %), cytoplasmic expression in 35 (31.5%), and nuclear accumulation in 15 (13.5%). E-cadherin alteration was observed in 34 cases (30.6%) and correlated with betacatenin alteration. On multivariate analysis, the combined immunoexpression group of beta-catenin nuclear accumulation/ E-cadherin alteration and the advanced TNM cancer stage group showed poor patient's survival (p<0.05). In conclusion, betacatenin activation through nuclear accumulation hardly occurred in EBV-infected gastric carcinomas. The combined immunoexpression pattern of beta-catenin and E-cadherin can be used as a prognostic marker in gastric carcinomas.
MeSH Terms
-
Adult
Aged
Cadherins/*metabolism
Carcinoma/*metabolism/*virology
Cell Nucleus/metabolism
Female
*Gene Expression Regulation, Neoplastic
*Gene Expression Regulation, Viral
Herpesvirus 4, Human/*metabolism
Humans
Immunohistochemistry/methods
In Situ Hybridization
Male
Middle Aged
NF-kappa B/metabolism
Prognosis
Stomach Neoplasms/*metabolism/*virology
beta Catenin/*metabolism
Figure
Reference
-
1. Plummer M, Franceschi S, Munoz N. Epidemiology of gastric cancer. IARC Sci Publ. 2004. 157:311–326.2. Cancer Registry system in Korea. Headquarters of Korea Central Cancer Registry. July 5, 2006. URL: http://ncc.re.kr.3. Kieff E, Rickinson AB. Fields BN, Knipe DM, Howley P, editors. Epstein-Barr virus and its replication. Fields virology. 2001. 4th ed. Philadelpia: Lippincott-Raven publishers;2511–2573.4. Becker KF, Keller G, Hoefler H. The use of molecular biology in diagnosis and prognosis of gastric cancer. Surg Oncol. 2000. 9:5–11.
Article5. Jawhari A, Jordan S, Poole S, Browne P, Pignatelli M, Farthing MJ. Abnormal immunoreactivity of the E-cadherin-catenin complex in gastric carcinoma: relationship with patient survival. Gastroenterology. 1997. 112:46–54.
Article6. Zhou YN, Xu CP, Han B, Li M, Qiao L, Fang DC, Yang JM. Expression of E-cadherin and beta-catenin in gastric carcinoma and its correlation with the clinicopathological features and patient survival. World J Gastroenterol. 2002. 8:987–993.7. Lee HS, Lee HK, Kim HS, Yang HK, Kim WH. Tumour suppressor gene expression correlates with gastric cancer prognosis. J Pathol. 2003. 200:39–46.
Article8. Ohno T, Aihara R, Kamiyama Y, Mochiki E, Asao T, Kuwano H. Prognostic significance of combined expression of MUC1 and adhesion molecules in advanced gastric cancer. Eur J Cancer. 2006. 42:256–263.
Article9. Deng J, Miller SA, Wang HY, Xia W, Wen Y, Zhou BP, Li Y, Lin SY, Hung MC. beta-catenin interacts with and inhibits NF-kappa B in human colon and breast cancer. Cancer Cell. 2002. 2:323–334.10. Chang MS, Lee HS, Jung EJ, Kim CW, Lee BL, Kim WH. Cell-cycle regulators, bcl-2 and NF-kappaB in Epstein-Barr virus-positive gastric carcinomas. Int J Oncol. 2005. 27:1265–1272.11. Polakis P. Wnt signaling and cancer. Genes Dev. 2000. 14:1837–1851.12. Barth AI, Nathke IS, Nelson WJ. Cadherins, catenins and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr Opin Cell Biol. 1997. 9:683–690.
Article13. Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999. 398:422–426.14. Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991. 251:1451–1455.
Article15. Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, Hofler H. E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res. 1994. 54:3845–3852.16. Gottardi CJ, Wong E, Gumbiner BM. E-cadherin suppresses cellular transformation by inhibiting beta-catenin signaling in an adhesion-independent manner. J Cell Biol. 2001. 153:1049–1060.17. Lipponen P, Saarelainen E, Ji H, Aaltomaa S, Syrjanen K. Expression of E-cadherin (E-CD) as related to other prognostic factors and survival in breast cancer. J Pathol. 1994. 174:101–109.
Article18. Bringuier PP, Umbas R, Schaafsma HE, Karthaus HF, Debruyne FM, Schalken JA. Decreased E-cadherin immunoreactivity correlates with poor survival in patients with bladder tumors. Cancer Res. 1993. 53:3241–3245.19. Umbas R, Isaacs WB, Bringuier PP, Schaafsma HE, Karthaus HF, Oosterhof GO, Debruyne FM, Schalken JA. Decreased E-cadherin expression is associated with poor prognosis in patients with prostate cancer. Cancer Res. 1994. 54:3929–3933.20. Krishnadath KK, Tilanus HW, van Blankenstein M, Hop WC, Kremers ED, Dinjens WN, Bosman FT. Reduced expression of the cadherin-catenin complex in oesophageal adenocarcinoma correlates with poor prognosis. J Pathol. 1997. 182:331–338.
Article21. Grabsch H, Takeno S, Noguchi T, Hommel G, Gabbert HE, Mueller W. Different patterns of beta-catenin expression in gastric carcinomas: relationship with clinicopathological parameters and prognostic outcome. Histopathology. 2001. 39:141–149.22. Nabais S, Machado JC, Lopes C, Seruca R, Carneiro F, Sobrinho-Simoes M. Different patterns of beta-catenin expression in gastric carcinomas: relationship with clinicopathological parameters and prognostic outcome. Histopathology. 2002. 41:368–369.23. Joo YE, Park CS, Kim HS, Choi SK, Rew JS, Kim SJ. Prognostic significance of E-cadherin/catenin complex expression in gastric cancer. J Korean Med Sci. 2000. 15:655–666.
Article24. Lim S, Lee HS, Kim HS, Kim YI, Kim WH. Alteration of E-cadherin-mediated adhesion protein is common, but microsatellite instability is uncommon in young age gastric cancers. Histopathology. 2003. 42:128–136.
Article25. Woo DK, Kim HS, Lee HS, Kang YH, Yang HK, Kim WH. Altered expression and mutation of beta-catenin gene in gastric carcinomas and cell lines. Int J Cancer. 2001. 95:108–113.26. Nabais S, Machado JC, Lopes C, Seruca R, Carneiro F, Sobrinho-Simoes M. Patterns of beta-catenin expression in gastric carcinoma: clinicopathological relevance and mutation analysis. Int J Surg Pathol. 2003. 11:1–9.27. Everly DN Jr, Kusano S, Raab-Traub N. Accumulation of cytoplasmic beta-catenin and nuclear glycogen synthase kinase 3beta in Epstein-Barr virus-infected cells. J Virol. 2004. 78:11648–11655.28. Huiping C, Kristjansdottir S, Jonasson JG, Magnusson J, Egilsson V, Ingvarsson S. Alterations of E-cadherin and beta-catenin in gastric cancer. BMC Cancer. 2001. 1:16.
Article29. Polakis P. The oncogenic activation of beta-catenin. Curr Opin Genet Dev. 1999. 9:15–21.30. Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinomas. Science. 1997. 275:1784–1787.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- E-cadherin and beta-catenin Expression and Mutation in Gastric Carcinomas
- Expression of E-cadherin and beta-catenin in Human Adenocarcinoma, Adenoma, and Ulcer of Stomach
- beta-Catenin Expression in Gastric Carcinogenesis
- The Role of E-cadherin/Beta-catenin Complex and Cyclin D1 in Head and Neck Squamous Cell Carcinoma
- RImmunohistochemical Evaluation of E-cadherin/catenin (alpha-, beta-, gamma-catenin and p120CTN) Complex Expression in Early Gastric Cancer