J Korean Med Sci.
2007 Feb;22(1):37-42. 10.3346/jkms.2007.22.1.37.
Eosinophil Cationic Protein and Chemokines in Nasopharyngeal Secretions of Infants with Respiratory Syncytial Virus (RSV) Bronchiolitis and Non-RSV Bronchiolitis
- Affiliations
-
- 1Department of Pediatrics, Kangnam St. Mary's Hospital College of Medicine, The Catholic University of Korea, Seoul, Korea. jslee@catholic.ac.kr
- KMID: 1713227
- DOI: http://doi.org/10.3346/jkms.2007.22.1.37
Abstract
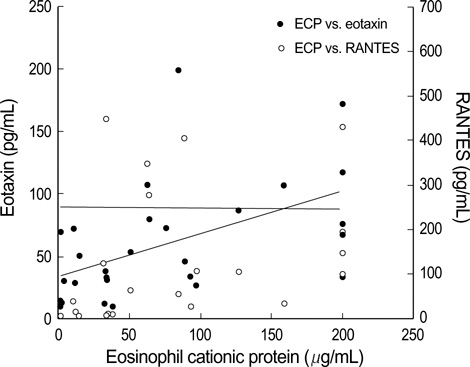
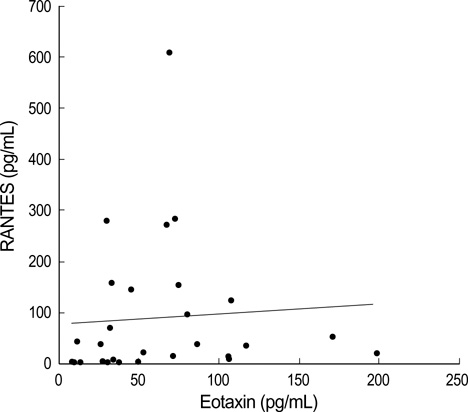
- Bronchiolitis is a risk factor for the development of childhood asthma. Eosinophilic inflammation in airways plays an important role in the pathophysiology of both bronchiolitis and asthma. To investigate this inflammation, we measured the eosinophil cationic protein (ECP), regulated on activation normal T-cell expressed and secreted (RANTES) and eotaxin levels in nasopharyngeal secretions (NPS). Twenty-eight patients with RSV bronchiolitis (RSV group), 11 patients with non-RSV bronchiolitis (non-RSV group) and 7 controls were enrolled in this study. ECP, RANTES, and eotaxin levels were measured by enzyme immunoassays. The ECP level in the NPS of the RSV group was significantly higher than that in the NPS of the non-RSV group and controls. RANTES and eotaxin levels in infants with bronchiolitis were significantly higher than those in the controls, but there was no significant difference between the RSV and non-RSV groups. In conclusion, with regard to eosinophilic airway inflammation, as compared with non-RSV bronchiolitis, RSV bronchiolitis may be more similar to childhood asthma.
Keyword
MeSH Terms
Figure
Reference
-
1. Sheeran P, Jafri H, Carubelli C, Saavedra J, Johnson C, Krisher K, Sandros PJ, Ramilo O. Elevated cytokine concentrations in the nasopharyngeal and tracheal secretions of children with respiratory syncytial virus disease. Pediatr Infect Dis J. 1999. 18:115–122.
Article2. Noah TL, Ivins SS, Murphy P, Kazachkova I, Moats-Staats B, Henderson FW. Chemokines and inflammation in the nasal passages of infants with respiratory syncytial virus bronchiolitis. Clin Immunol. 2002. 104:86–95.
Article3. Schwarze J, Hamelmann E, Bradley KL, Takeda K, Gelfand EW. Respiratory syncytial virus infection results in airway hyperresponsiveness and enhanced airway sensitization to allergen. J Clin Invest. 1997. 100:226–233.
Article4. Greenough A. Respiratory syncytial virus infection: clinical features, management, and prophylaxis. Curr Opin Pulm Med. 2002. 8:214–217.
Article5. Wang SZ, Forsyth KD. The interaction of neutrophils with respiratory epithelial cells in viral infection. Respirology. 2000. 5:1–10.
Article6. Byington CL, Castillo H, Gerber K, Daly JA, Brimley LA, Adams S, Christenson JS, Pavia AT. The effect of rapid respiratory viral diagnostic testing on antibiotic use in a children's hospital. Arch Pediatr Adolesc Med. 2002. 156:1230–1234.
Article7. Olszewska-Pazdrak B, Casola A, Saito T, Alam R, Crowe SE, Mei F, Ogra PL, Garofalo RP. Cell-specific expression of RANTES, MCP-1, and MIP-1-alpha by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. J Virol. 1998. 72:4756–4764.8. Harrison AM, Bonville CA, Rosenberg HF, Domachowske JB. Respiratory syncytial virus-induced chemokine expression in the lower airways. Am J Respir Crit Care Med. 1999. 159:1918–1924.9. Hornsleth A, Loland L, Larsen LB. Cytokines and chemokines in respiratory secretion and severity of disease in infants with respiratory syncytial virus (RSV) infection. J Clin Virol. 2001. 21:163–170.
Article10. Garofalo R, Dorris A, Ahlstedt S, Welliver RC. Peripheral blood eosinophil counts and eosinophil cationic protein content of respiratory secretions in bronchiolitis: relationship to severity of disease. Pediatr Allergy Immunol. 1994. 5:111–117.
Article11. Colocho Zelaya EA, Orvell C, Strannegard O. Eosinophil cationic protein in nasopharyngeal secretions and serum of infants infected with respiratory syncytial virus. Pediatr Allergy Immunol. 1994. 5:100–106.
Article12. Pifferi M, Ragazzo V, Caramella D, Baldini G. Eosinophil cationic protein in infants with respiratory syncytial virus bronchiolitis: predictive value for subsequent development of persistent wheezing. Pediatr Pulmonol. 2001. 31:419–424.
Article13. Venge P. Role of eosinophils in childhood asthma inflammation. Pediatr Pulmonol Suppl. 1995. 11:34–35.
Article14. Sung RY, Hui SH, Wong CK, Lam CW, Yin J. A comparison of cytokine responses in respiratory syncytial virus and inflenza A infections in infants. Eur J Pediatr. 2001. 160:117–122.15. Chung HL, Kim SG. RANTES may be predictive of later recurrent wheezing after respiratory syncytial virus bronchiolitis in infants. Ann Allergy Asthma Immunol. 2002. 88:463–467.
Article16. Oh JW, Lee HB, Park IK, Kang JO. Interleukin-6, interleukin-8, interleukin-11, and interferon-gamma levels in nasopharyngeal aspirates from wheezing children with respiratory syncytial virus or influenza A virus infection. Pediatr Allergy Immunol. 2002. 13:350–356.17. Pizzichini E, Pizzichini MM, Efthimiadis A, Dolovich J, Hargreave FE. Measuring airway inflammation in asthma: eosinophils and eosinophilic cationic protein in induced sputum compared with peripheral blood. J Allergy Clin Immunol. 1997. 99:539–544.
Article18. Rosi E, Ronchi MC, Grazzini M, Duranti R, Scano G. Diagnostic accuracy of sputum outcomes in chronic stable asthma. Clin Exp Allergy. 2000. 30:577–584.
Article19. Shields MD, Brown V, Stevenson EC, Fitch PS, Schock BC, Turner G, Taylor R, Ennis M. Serum eosinophil cationic protein and blood eosinophil counts for the prediction of the presence of airways inflammation in children with wheezing. Clin Exp Allergy. 1999. 29:1382–1389.20. Ponath PD, Qin S, Ringler DJ, Clark-Lewis I, Wang J, Kassam N, Smith H, Shi X, Gonzalo JA, Newman W, Gutierrez-Ramos JC, Mackay CR. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J Clin Invest. 1996. 97:604–612.
Article21. Hossny E, Aboul-Magd M, Bakr S. Increased plasma eotaxin in atopic dermatitis and acute urticaria in infants and children. Allergy. 2001. 56:996–1002.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- ECP, RANTES, and Eotaxin in Respiratory Syncytial Virus Bronchiolitis: Relation to Subsequent Wheezing
- The Effect of Respiratory Syncytial Virus Infection on the Production of Interleukin-5 and RANTES in Bronchiolitis
- Eosinophil Activation Induced by RANTES (Regulated upon Activation, Normal T-Cell-Expressed-Secreted) in Respiratory Syncytial Virus Bronchiolitis
- The Role of TNF-alpha in Eosinophilic Inflammation of RSV Bronchiolitis
- IL-11, IFN-gamma and ECP levels in nasopharyngeal secretions from non-asthmatic wheezing children with respiratory syncytial virus or influenza A virus infections