J Vet Sci.
2013 Dec;14(4):473-479. 10.4142/jvs.2013.14.4.473.
Distribution and accumulation of Cy5.5-labeled thermally cross-linked superparamagnetic iron oxide nanoparticles in the tissues of ICR mice
- Affiliations
-
- 1College of Veterinary Medicine and Research Institute of Veterinary Medicine, Chungbuk National University, Cheongju 361-763, Korea. beomjun@cbu.ac.kr
- 2College of Veterinary Medicine and Institute of Life Science, Gyeongsang National University, Jinju 600-701, Korea.
- 3School of Life Science, Gwangju Institute of Science and Technology, Gwangju 500-712, Korea.
- KMID: 1712315
- DOI: http://doi.org/10.4142/jvs.2013.14.4.473
Abstract
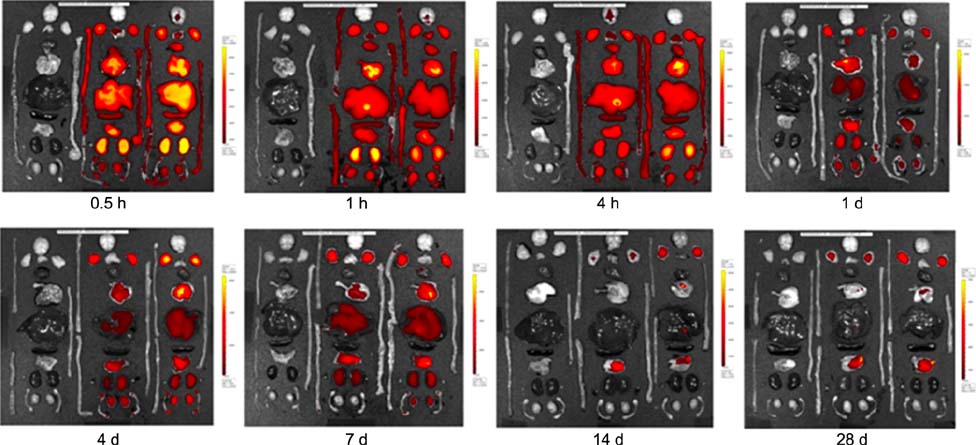
- Free Cy5.5 dye and Cy5.5-labeled thermally cross-linked superparamagnetic iron oxide nanoparticles (TCL-SPION) have been routinely used for in vivo optical imaging. However, there is little information about the distribution and accumulation of free Cy5.5 dye and Cy5.5-labeled TCL-SPION in the tissues of mice. Free Cy5.5 dye (0.1 mg/kg body weight) and Cy5.5-labeled TCL-SPION (15 mg/kg body weight) were intravenously injected into the tail vein of ICR mice. The biodistribution and accumulation of the TCL-SPION and Cy5.5 were observed by ex vivo optical imaging and fluorescence signal generation at various time points over 28 days. Cy5.5 dye fluorescence in various organs was rapidly eliminated from 0.5 to 24 h post-injection. Fluorescence intensity of Cy5.5 dye in the liver, lung, kidney, and stomach was fairly strong at the early time points within 1 day post-injection. Cy5.5-labeled TCL-SPION had the highest fluorescence density in the lung at 0.5 h post-injection and decreased rapidly over time. Fluorescence density in liver and spleen was maintained over 28 days. These results suggest that TCL-SPION can be useful as a carrier of therapeutic reagents to treat diseases by persisting for long periods of time in the body.
Keyword
MeSH Terms
Figure
Reference
-
1. Alexiou C, Arnold W, Klein RJ, Parak FG, Hulin P, Bergemann C, Erhardt W, Wagenpfeil S, Lübbe AS. Locoregional cancer treatment with magnetic drug targeting. Cancer Res. 2000; 60:6641–6648.2. Alexiou C, Schmid RJ, Jurgons R, Kremer M, Wanner G, Bergemann C, Huenges E, Nawroth T, Arnold W, Parak FG. Targeting cancer cells: magnetic nanoparticles as drug carriers. Eur Biophys J. 2006; 35:446–450.
Article3. Briley-Saebo KC, Johansson LO, Hustvedt SO, Haldorsen AG, Bjørnerud A, Fayad ZA, Ahlstrom HK. Clearance of iron oxide particles in rat liver: effect of hydrated particle size and coating material on liver metabolism. Invest Radiol. 2006; 41:560–571.4. Bulte JWM, Zhang SC, van Gelderen P, Herynek V, Jordan EK, Duncan ID, Frank JA. Neurotransplantation of magnetically labeled oligodendrocyte progenitors: magnetic resonance tracking of cell migration and myelination. Proc Natl Acad Sci U S A. 1999; 96:15256–15261.
Article5. Chapman G, Henary M, Patonay G. The effect of varying short-chain alkyl substitution on the molar absorptivity and quantum yield of cyanine dyes. Anal Chem Insights. 2011; 6:29–36.
Article6. Chastellain M, Petri A, Gupta A, Rao KV, Hofmann H. Super paramagnetic silica-iron oxide nanocomposites for application in hyperthermia. Adv Eng Mater. 2004; 6:235–241.
Article7. Cho WS, Cho M, Jeong J, Choi M, Cho HY, Han BS, Kim SH, Kim HO, Lim YT, Chung BH, Jeong J. Acute toxicity and pharmacokinetics of 13 nm-sized PEG-coated gold nanoparticles. Toxicol Appl Pharmacol. 2009; 236:16–24.
Article8. Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nat Biotechnol. 2007; 25:1165–1170.
Article9. Cunningham BT. Photonic crystal surfaces as a general purpose platform for label-free and fluorescent assays. JALA Charlottesv Va. 2010; 15:120–135.
Article10. Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005; 5:161–171.
Article11. Gupta AK, Curtis ASG. Surface modified superparamagnetic nanoparticles for drug delivery: interaction studies with human fibroblasts in culture. J Mater Sci Mater Med. 2004; 15:493–496.
Article12. Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005; 26:3995–4021.
Article13. Hara K, Tsujimoto H, Tsukada Y, Huang CC, Kawashima Y, Tsutsumi M. Histological examination of PLGA nanospheres for intratracheal drug administration. Int J Pharm. 2008; 356:267–273.
Article14. Horák D, Rittich B, Španová A, Beneš MJ. Magnetic microparticulate carriers with immobilized selective ligands in DNA diagnostics. Polymer. 2005; 46:1245–1255.
Article15. Jain KK. Role of nanobiotechnology in developing personalized medicine for cancer. Technol Cancer Res Treat. 2005; 4:645–650.
Article16. Jordan A, Scholz R, Maier-Hauff K, Johannsen M, Wust P, Nadobny J, Schirra H, Schmidt H, Deger S, Loening S, Lanksch W, Felix R. Presentation of a new magnetic field therapy system for the the treatment of human solid tumors with magnetic fluid hyperthermia. J Magn Magn Mater. 2001; 225:118–126.
Article17. Josephson L, Tung CH, Moore A, Weissleder R. High-efficiency intracellular magnetic labeling with novel superparamagnetic-tat peptide conjugates. Bioconjug Chem. 1999; 10:186–191.
Article18. Kohler N, Sun C, Fichtenholtz A, Gunn J, Fang C, Zhang M. Methotrexate-immobilized poly(ethylene glycol) magnetic nanoparticles for MR imaging and drug delivery. Small. 2006; 2:785–792.
Article19. Kwon JT, Hwang SK, Jin H, Kim DS, Minai-Tehrani A, Yoon HJ, Choi M, Yoon TJ, Han DY, Kang YW, Yoon BI, Lee JK, Cho MH. Body distribution of inhaled fluorescent magnetic nanoparticles in the mice. J Occup Health. 2008; 50:1–6.
Article20. Lee CM, Jeong HJ, Yun KN, Kim DW, Sohn MH, Lee JK, Jeong J, Lim ST. Optical imaging to trace near infrared fluorescent zinc oxide nanoparticles following oral exposure. Int J Nanomedicine. 2012; 7:3203–3209.21. Lee H, Lee E, Kim DK, Jang NK, Jeong YY, Jon S. Antibiofouling polymer-coated superparamagnetic iron oxide nanoparticles as potential magnetic resonance contrast agents for in vivo cancer imaging. J Am Chem Soc. 2006; 128:7383–7389.
Article22. Lee H, Yu MK, Park S, Moon S, Min JJ, Jeong YY, Kang HW, Jon S. Thermally cross-linked superparamagnetic iron oxide nanoparticles: synthesis and application as a dual imaging probe for cancer in vivo. J Am Chem Soc. 2007; 129:12739–12745.
Article23. Lee JH, Huh YM, Jun Y, Seo J, Jang J, Song HT, Kim S, Cho EJ, Yoon HG, Suh JS, Cheon J. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat Med. 2007; 13:95–99.
Article24. Lewin M, Carlesso N, Tung CH, Tang XW, Cory D, Scadden DT, Weissleder R. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol. 2000; 18:410–414.
Article25. Lübbe AS, Bergemann C, Riess H, Schriever F, Reichardt P, Possinger K, Matthias M, Dörken B, Herrmann F, Gürtler R, Hohenberger P, Haas N, Sohr R, Sander B, Lemke AJ, Ohlendorf D, Huhnt W, Huhn D. Clinical experiences with magnetic drug targeting: a phase I study with 4'-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res. 1996; 56:4686–4693.26. Neuberger T, Schöpf B, Hofmann H, Hofmann M, von Rechenberg B. Superparamagnetic nanoparticles for biomedical applications: possibilities and limitations of a new drug delivery system. J Magn Magn Mater. 2005; 293:483–496.
Article27. Rosenholm JM, Mamaeva V, Sahlgren C, Lindén M. Nanoparticles in targeted cancer therapy: mesoporous silica nanoparticles entering preclinical development stage. Nanomedicine (Lond). 2012; 7:111–120.
Article28. Snoeks TJA, Löwik CWGM, Kaijzel EL. 'In vivo' optical approaches to angiogenesis imaging. Angiogenesis. 2010; 13:135–147.29. Tartaj P, Morales MP, Veintemillas-Verdaguer S, González-Carreño T, Serna CJ. The preparation of magnetic nanoparticles for applications in biomedicine. J Phys D appl Phys. 2003; 36:R182–R197.30. Thomas R, Park IK, Jeong YY. Magnetic iron oxide nanoparticles for multimodal imaging and therapy of cancer. Int J Mol Sci. 2013; 14:15910–15930.
Article31. Thorek DLJ, Chen AK, Czupryna J, Tsourkas A. Superparamagnetic iron oxide nanoparticle probes for molecular imaging. Ann Biomed Eng. 2006; 34:23–38.
Article32. Wang F, Tan WB, Zhang Y, Fan X, Wang M. Luminescent nanomaterials for biological labeling. Nanotechnology. 2006; 17:R1–R13.33. Wang X, Zhang R, Wu C, Dai Y, Song M, Gutmann S, Gao F, Lv G, Li J, Li X, Guan Z, Fu D, Chen B. The application of Fe3O4 nanoparticles in cancer research: a new strategy to inhibit drug resistance. J Biomed Mater Res A. 2007; 80:852–860.34. Willard MA, Kurihara LK, Carpenter EE, Calvin S, Harris VG. Chemically prepared magnetic nanoparticles. Int Mater Rev. 2004; 49:125–170.
Article35. Yang RSH, Chang LW, Wu JP, Tsai MH, Wang HJ, Kuo YC, Yeh TK, Yang CS, Lin P. Persistent tissue kinetics and redistribution of nanoparticles, quantum dot 705, in mice: ICP-MS quantitative assessment. Environ Health Perspect. 2007; 115:1339–1343.
Article36. Yu MK, Jeong YY, Park J, Park S, Kim JW, Min JJ, Kim K, Jon S. Drugloaded superparamagnetic iron oxide nanoparticles for combined cancer imaging and therapy in vivo. Angew Chem Int Ed Engl. 2008; 47:5362–5365.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Distribution and accumulation of 177Lu-labeled thermally cross-linked superparamagnetic iron oxide nanoparticles in the tissues of ICR mice
- Evaluation of thermally cross-linked superparamagnetic iron oxide nanoparticles for the changes of concentration and toxicity on tissues of Sprague-Dawley rats
- Labeling Efficacy of Superparamagnetic Iron Oxide Nanoparticles to Human Neural Stem Cells: Comparison of Ferumoxides, Monocrystalline Iron Oxide, Cross-linked Iron Oxide (CLIO)-NH2 and tat-CLIO
- Evaluation of Porcine Pancreatic Islets Transplanted in the Kidney Capsules of Diabetic Mice Using a Clinically Approved Superparamagnetic Iron Oxide (SPIO) and a 1.5T MR Scanner
- In vivo Tracking of Mesenchymal Stem Cells Labeled with a Novel Chitosan-coated Superparamagnetic Iron Oxide Nanoparticles using 3.0T MRI