Korean J Radiol.
2010 Dec;11(6):673-682. 10.3348/kjr.2010.11.6.673.
Evaluation of Porcine Pancreatic Islets Transplanted in the Kidney Capsules of Diabetic Mice Using a Clinically Approved Superparamagnetic Iron Oxide (SPIO) and a 1.5T MR Scanner
- Affiliations
-
- 1Department of Radiology, Seoul National University Hospital, Seoul 110-744, Korea. moonwk@snu.ac.kr
- 2Institute of Radiation Medicine, Medical Research Center, Seoul National University, Seoul 110-744, Korea.
- 3Department of Internal Medicine, Seoul National University Hospital, Seoul 110-744, Korea.
- KMID: 1119231
- DOI: http://doi.org/10.3348/kjr.2010.11.6.673
Abstract
OBJECTIVE
To evaluate transplanted porcine pancreatic islets in the kidney capsules of diabetic mice using a clinically approved superparamagnetic iron oxide (SPIO) and a 1.5T MR scanner.
MATERIALS AND METHODS
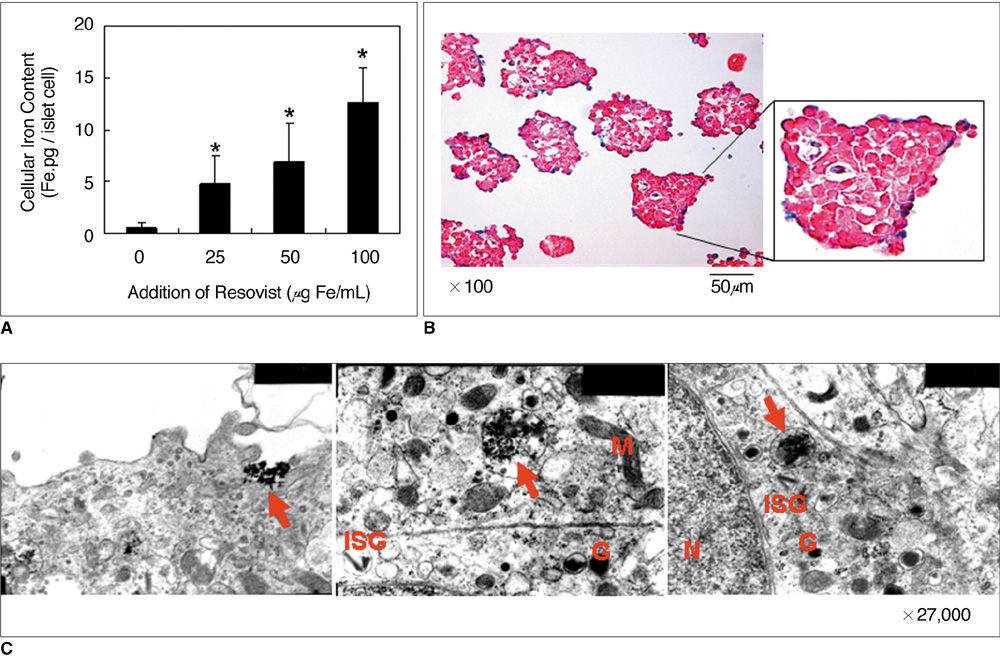
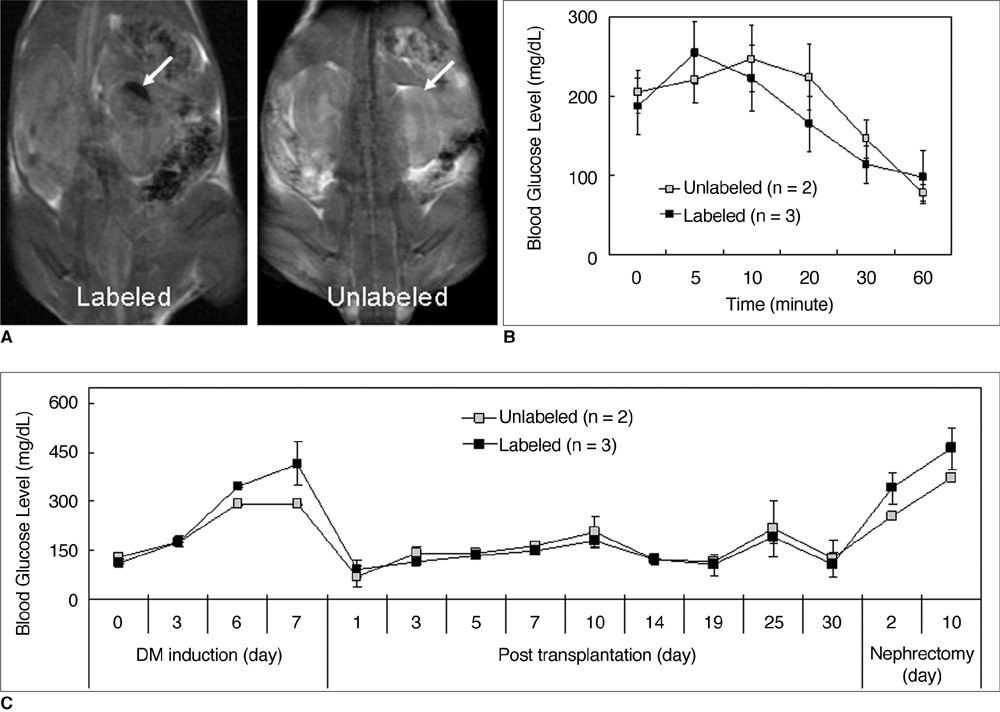
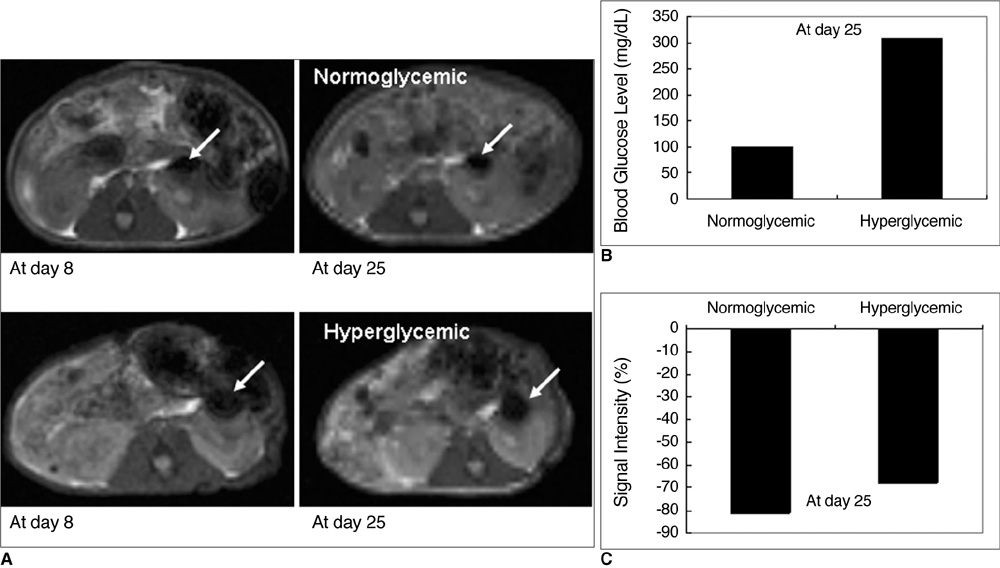
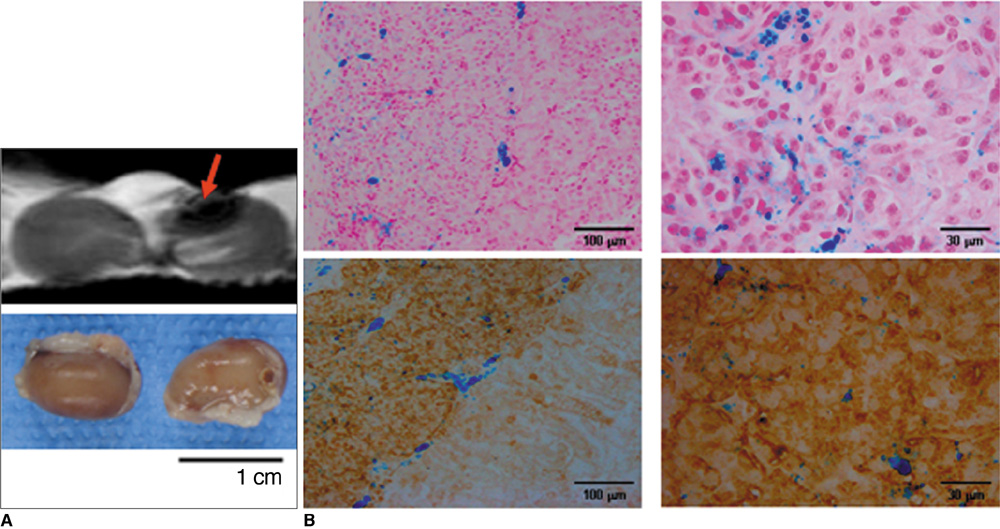
Various numbers of porcine pancreatic islets labeled with Resovist, a carboxydextran-coated SPIO, were transplanted into the kidney capsules of normal mice and imaged with a 3D FIESTA sequence using a 1.5T clinical MR scanner. Labeled (n = 3) and unlabeled (n = 2) islets were transplanted into the kidney capsules of streptozotocin-induced diabetic mice. Blood glucose levels and MR signal intensities were monitored for 30 days post-transplantation.
RESULTS
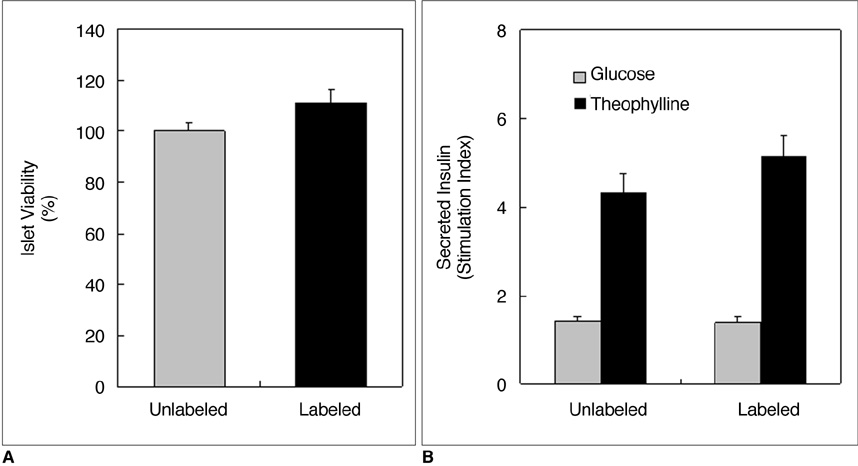
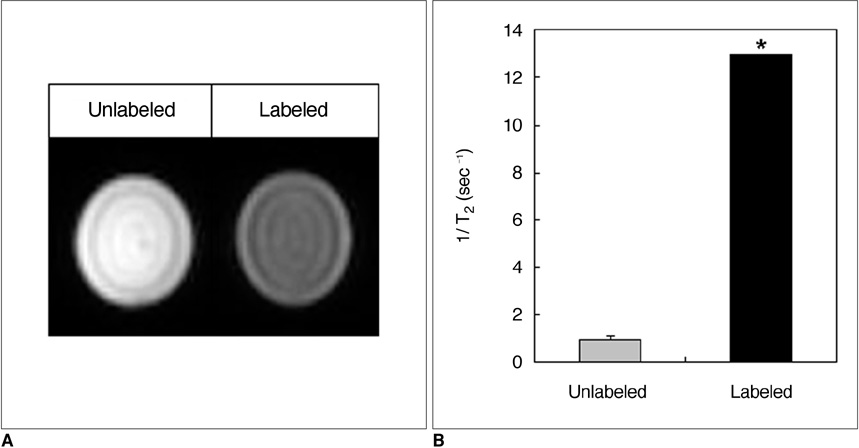
There were no significant differences in viability or insulin secretion between labeled and unlabeled islets. A strong correlation (r2 > 0.94) was evident between the number of transplanted islets and T2 relaxation times quantified by MRI. Transplantation with labeled or unlabeled islets helped restore normal sustained glucose levels in diabetic mice, and nephrectomies induced the recurrence of diabetes. The MR signal intensity of labeled pancreatic islets decreased by 80% over 30 days.
CONCLUSION
The transplantation of SPIO-labeled porcine islets into the kidney capsule of diabetic mice allows to restore normal glucose levels, and these islets can be visualized and quantified using a 1.5T clinical MR scanner.
Keyword
MeSH Terms
Figure
Reference
-
1. Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000. 343:230–238.2. Shapiro AM, Ricordi C, Hering B. Edmonton's islet success has indeed been replicated elsewhere. Lancet. 2003. 362:1242.3. Kim SJ, Doudet DJ, Studenov AR, Nian C, Ruth TJ, Gambhir SS, et al. Quantitative micro positron emission tomography (PET) imaging for the in vivo determination of pancreatic islet graft survival. Nat Med. 2006. 12:1423–1428.4. Evgenov NV, Medarova Z, Dai G, Bonner-Weir S, Moore A. In vivo imaging of islet transplantation. Nat Med. 2006. 12:144–148.5. Elliott RB, Escobar L, Tan PL, Garkavenko O, Calafiore R, Basta P, et al. Intraperitoneal alginate-encapsulated neonatal porcine islets in a placebo-controlled study with 16 diabetic cynomolgus primates. Transplant Proc. 2005. 37:3505–3508.6. Cozzi E, Bosio E. Islet xenotransplantation: current status of preclinical studies in the pig-to-nonhuman primate model. Curr Opin Organ Transplant. 2008. 13:155–158.7. Moore A, Marecos E, Bogdanov A Jr, Weissleder R. Tumoral distribution of long-circulating dextran-coated iron oxide nanoparticles in a rodent model. Radiology. 2000. 214:568–574.8. Lewin M, Carlesso N, Tung CH, Tang XW, Cory D, Scadden DT, et al. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol. 2000. 18:410–414.9. Bulte JW, Zhang S, van Gelderen P, Herynek V, Jordan EK, Duncan ID, et al. Neurotransplantation of magnetically labeled oligodendrocyte progenitors: magnetic resonance tracking of cell migration and myelination. Proc Natl Acad Sci U S A. 1999. 96:15256–15261.10. Dodd CH, Hsu HC, Chu WJ, Yang P, Zhang HG, Mountz JD Jr, et al. Normal T-cell response and in vivo magnetic resonance imaging of T-cells loaded with HIV transactivator-peptide-derived superparamagnetic nanoparticles. J Immunol Methods. 2001. 256:89–105.11. Dousset V, Delalande C, Ballarino L, Quesson B, Seilhan D, Coussemacq M, et al. In vivo macrophage activity imaging in the central nervous system detected by magnetic resonance. Magn Reson Med. 1999. 41:329–333.12. Jung SI, Kim SH, Kim HC, Son KR, Chung SY, Moon WK, et al. In vivo MR imaging of magnetically labeled mesenchymal stem cells in a rat model of renal ischemia. Korean J Radiol. 2009. 10:277–284.13. Jirák D, Kríz J, Herynek V, Andersson B, Girman P, Burian M, et al. MRI of transplanted pancreatic islets. Magn Reson Med. 2004. 52:1228–1233.14. Shapiro AM, Hao EG, Lakey JR, Yakimets WJ, Churchill TA, Mitlianga PG, et al. Novel approaches toward early diagnosis of islet allograft rejection. Transplantation. 2001. 71:1709–1718.15. Evgenov NV, Medarova Z, Pratt J, Pantazopoulos P, Leyting S, Bonner-Weir S, et al. In vivo imaging of immune rejection in transplanted pancreatic islets. Diabetes. 2006. 55:2419–2428.16. Tai JH, Foster P, Rosales A, Feng B, Hasilo C, Martinez V, et al. Imaging islets labeled with magnetic nanoparticles at 1.5 Tesla. Diabetes. 2006. 55:2931–2938.17. Symonds P, Murray JC, Hunter AC, Debska G, Szewczyk A, Moghimi SM. Low and high molecular weight poly(L-lysine)s/poly(L-lysine)-DNA complexes initiate mitochondrial-mediated apoptosis differently. FEBS Lett. 2005. 579:6191–6198.18. Kim HS, Choi Y, Song IC, Moon WK. Magnetic resonance imaging and biological properties of pancreatic islets labeled with iron oxide nanoparticles. NMR Biomed. 2009. 22:852–856.19. Matuszewski L, Persigehl T, Wall A, Schwindt W, Tombach B, Fobker M, et al. Cell tagging with clinically approved iron oxides: feasibility and effect of lipofection, particle size, and surface coating on labeling efficiency. Radiology. 2005. 235:155–161.20. Kim JH, Kim HI, Lee KW, Yu JE, Kim SH, Park HS, et al. Influence of strain and age differences on the yields of porcine islet isolation: extremely high islet yields from SPF CMS miniature pigs. Xenotransplantation. 2007. 14:60–66.21. van der Burg MP, Basir I, Bouwman E. No porcine islet loss during density gradient purification in a novel iodixanol in University of Wisconsin solution. Transplant Proc. 1998. 30:362–363.22. Reimer P, Balzer T. Ferucarbotran (Resovist): a new clinically approved RES-specific contrast agent for contrast-enhanced MRI of the liver: properties, clinical development, and applications. Eur Radiol. 2003. 13:1266–1276.23. Berkova Z, Kriz J, Girman P, Zacharovova K, Koblas T, Dovolilova E, et al. Vitality of pancreatic islets labeled for magnetic resonance imaging with iron particles. Transplant Proc. 2005. 37:3496–3498.24. Evgenov NV, Pratt J, Pantazopoulos P, Moore A. Effects of glucose toxicity and islet purity on in vivo magnetic resonance imaging of transplanted pancreatic islets. Transplantation. 2008. 85:1091–1098.25. Jirak D, Kriz J, Strzelecki M, Yang J, Hasilo C, White DJ, et al. Monitoring the survival of islet transplants by MRI using a novel technique for their automated detection and quantification. MAGMA. 2009. 22:257–265.26. Kriz J, Jirák D, Girman P, Berková Z, Zacharovova K, Honsova E, et al. Magnetic resonance imaging of pancreatic islets in tolerance and rejection. Transplantation. 2005. 80:1596–1603.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Histologic Monitoring of the Transplanted Superparamagnetic Iron Oxide Labelled Human Mesenchymal Stem Cells in the Rat Bladder
- Usefulness of Superparamagnetic Iron Oxide (SPIO) as a Negative Oral Contrast Agent in MR Cholangiopancreatography
- Monitoring Transplanted Human Mesenchymal Stem Cells in the Penile Cavernosal Tissues of Streptozotocin-induced Diabetic Rats Using Molecular Magnetic Resonance Imaging
- The Impact of the Amount of Intracellular SPIO on MR Signal Intensity during In Vivo Tracking of Macrophage Homing
- Molecular MRI Images of the Transplanted Human Mesenchymal Stem Cells in the Liver, Kidney, Bladder and Penile Cavernosum of Rats