J Vet Sci.
2013 Sep;14(3):263-270. 10.4142/jvs.2013.14.3.263.
Expression of verocytotoxic Escherichia coli antigens in tobacco seeds and evaluation of gut immunity after oral administration in mouse model
- Affiliations
-
- 1Universita di Milano, Department of Health, Animal Science and Food Safety, 20134 Milan, Italy. luciana.rossi@unimi.it
- 2Plantechno S.r.l., 26040 Vicomoscano, Cremona, Italy.
- 3Universita di Milano, Department of Animal Pathology, Hygiene and Veterinary Public Health, 20133 Milan, Italy.
- 4Laboratory of Immunology, Faculty of Veterinary Medicine, Ghent University, 9820 Merelbeke, Belgium.
- 5Catholic University, Institute of Agronomy, Genetics and Field Crops, 29100 Piacenza, Italy.
- KMID: 1705556
- DOI: http://doi.org/10.4142/jvs.2013.14.3.263
Abstract
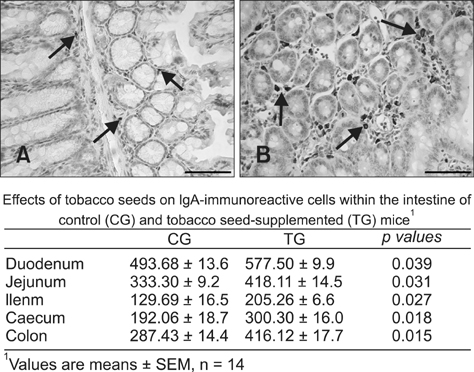
- Verocytotoxic Escherichia (E.) coli strains are responsible for swine oedema disease, which is an enterotoxaemia that causes economic losses in the pig industry. The production of a vaccine for oral administration in transgenic seeds could be an efficient system to stimulate local immunity. This study was conducted to transform tobacco plants for the seed-specific expression of antigenic proteins from a porcine verocytotoxic E. coli strain. Parameters related to an immunological response and possible adverse effects on the oral administration of obtained tobacco seeds were evaluated in a mouse model. Tobacco was transformed via Agrobacteium tumefaciens with chimeric constructs containing structural parts of the major subunit FedA of the F18 adhesive fimbriae and VT2e B-subunit genes under control of a seed specific GLOB promoter. We showed that the foreign Vt2e-B and F18 genes were stably accumulated in storage tissue by the immunostaining method. In addition, Balb-C mice receiving transgenic tobacco seeds via the oral route showed a significant increase in IgA-positive plasma cell presence in tunica propria when compared to the control group with no observed adverse effects. Our findings encourage future studies focusing on swine for evaluation of the protective effects of transformed tobacco seeds against E. coli infection.
MeSH Terms
-
Administration, Oral
Agrobacterium tumefaciens
Animals
Antigens, Bacterial/genetics/metabolism
Bacterial Vaccines/administration & dosage/adverse effects/*pharmacology
Edema Disease of Swine/*immunology/microbiology
Escherichia coli Infections/immunology/microbiology/*veterinary
Escherichia coli Proteins/*genetics/metabolism
Female
Fimbriae Proteins/genetics/metabolism
Genetic Engineering
Intestines/immunology/microbiology/pathology
Mice
Mice, Inbred BALB C
Models, Animal
Plants, Genetically Modified/*genetics/metabolism
Seeds/genetics/metabolism
Shiga Toxin 2/genetics/metabolism
Shiga-Toxigenic Escherichia coli/genetics/immunology/*pathogenicity
Swine
Tobacco/*genetics/metabolism
Virulence Factors/genetics/metabolism
Antigens, Bacterial
Bacterial Vaccines
Escherichia coli Proteins
Fimbriae Proteins
Shiga Toxin 2
Virulence Factors
Figure
Reference
-
1. Cheng D, Sun H, Xu J, Gao S. Prevalence of fimbial colonization factors F18ab and F18ac in Escherichia coli isolates from weaned piglets with edema and/or diarrhea in China. Vet Microbiol. 2005; 110:35–39.
Article2. Chen X, Gao S, Jiao X, Liu XF. Prevalence of serogroups and virulence factors of Escherichia coli strains isolated from pigs with postweaning diarrhoea in eastern China. Vet Microbiol. 2004; 103:13–20.
Article3. Coddens A, Diswall M, Ångström J, Brelmer ME, Goddeeris B, Cox E, Teneberg S. Recognition of blood group ABH type 1 determinants by the FedF adhesin of F18-fimbriated Escherichia coli. J Biol Chem. 2009; 284:9713–9726.
Article4. Daniell H, Singh ND, Mason H, Streatfield SJ. Plant-made vaccine antigens and biopharmaceuticals. Trends Plant Sci. 2009; 14:669–679.
Article5. Di Giancamillo A, Vitari F, Bosi G, Savoini G, Domeneghini C. The chemical code of porcine enteric neurons and the number of enteric glial cells are altered by dietary probiotics. Neurogastroenterol Motil. 2010; 22:e271–e278.
Article6. Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987; 19:11–15.7. Fagan PK, Hornitzky MA, Bettelheim KA, Djordjevic SP. Detection of Shiga-like toxin (stx1 and stx2), intimin (eaeA) and enterohemorrhagic Escherichia coli (EHEC) hemolysin (EHEC hlyA) genes in animal feces by multiplex PCR. Appl Environ Microbiol. 1999; 65:868–872.
Article8. Fischer R, Stoger E, Schillberg S, Christou P, Twyman RM. Plant-based production of biopharmaceuticals. Curr Opin Plant Biol. 2004; 7:152–158.
Article9. Floss DM, Falkenburg D, Conrad U. Production of vaccines and therapeutic antibodies for veterinary applications in transgenic plants: an overview. Transgenic Res. 2007; 16:315–332.
Article10. Franke S, Gunzer F, Wieler LH, Baljer G, Karch H. Construction of recombinant Shiga-like toxin-IIv (SLT-IIv) and its use in monitoring the SLT-IIv antibody status of pigs. Vet Microbiol. 1995; 43:41–52.
Article11. Giddings G, Allison G, Brooks D, Carter A. Transgenic plants as factories for biopharmaceuticals. Nat Biotechnol. 2000; 18:1151–1155.
Article12. Hong TTT, Linh NQ, Ogle B, Lindberg JE. Survey on the prevalence of diarrhoea in pre-weaning piglets and on feeding systems as contributing risk factors in smallholdings in Central Vietnam. Trop Anim Health Prod. 2006; 38:397–405.
Article13. Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Roger SG, Fraley RT. A simple and general method for transferring genes into plants. Science. 1985; 227:1229–1231.
Article14. Imberechts H, De Greve H, Lintermans P. The pathogenesis of edema disease in pigs. Vet Microbiol. 1992; 31:221–233.15. Kolotilin I, Kaldis A, Devriendt B, Joensuu J, Cox E, Menassa R. Production of a subunit vaccine candidate against porcine post-weaning diarrhea in high-biomass transplastomic tobacco. PLoS One. 2012; 7:e42405.
Article16. Mason HS, Warzecha H, Mor T, Arntzen CJ. Edible plant vaccines: applications for prophylactic and therapeutic molecular medicine. Trends Mol Med. 2002; 8:324–329.
Article17. Nochi T, Takagi H, Yuki Y, Yang L, Masumura T, Mejima M, Nakanishi U, Matsumura A, Uozumi A, Hiroi T, Morita S, Tanaka K, Takaiwa F, Kiyono H. Rice-based mucosal vaccine as a global strategy for cold-chain- and needle-free vaccination. Proc Natl Acad Sci U S A. 2007; 104:10986–10991.
Article18. O'Brien AD, Holmes RK. Shiga and Shiga-like toxins. Microbiol Rev. 1987; 51:206–220.19. Reggi S, Marchetti S, Patti T, De Amicis F, Cariati R, Bembi B, Fogher C. Recombinant human acid β-glucosidase stored in tobacco seed is stable, active and taken up by human fibroblasts. Plant Mol Biol. 2005; 57:101–113.
Article20. Rippinger P, Bertschinger HU, Imberechts H, Nagy B, Sorg I, Stamm M, Wild P, Wittig W. Designations F18ab and F18ac for the related fimbrial types F107, 2134P and 8813 of Escherichia coli isolated from porcine postweaning diarrhoea and from oedema disease. Vet Microbiol. 1995; 45:281–295.
Article21. Rossi L, Reggi S, Di Giancamillo A, Domeneghini C, Pinotti L, Fogher C, Baldi A. Oral administration of tobacco seeds expressing antigenic proteins in mice Balb-C: a model of edile vaccines for oedema disease. Ital J Anim Sci. 2003; 2:Suppl. 7–9.22. Rossi L, Baldi A, Dell'Orto V, Reggi S, Fogher C. Expression of flak flagellin from Salmonella Typhimurium in tobacco seeds. IOSRJP. 2012; 2:19–22.
Article23. Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. 3rd ed. New York: Cold Spring Harbour Laboratory Press;2001. p. 7.31–7.44.24. Santi L, Giritch A, Roy CJ, Marillonnet S, Klimyuk V, Gleba Y, Webb R, Arntzen CJ, Mason HS. Protection conferred by recombinant Yersinia pestis antigens produced by a rapid and highly scalable plant expression system. Proc Natl Acad Sci U S A. 2006; 103:861–866.
Article25. Sarrazin E, Bertschinger HU. Role of fimbriae F18 for actively acquired immunity against porcine enterotoxigenic Escherichia coli. Vet Microbiol. 1997; 54:133–144.
Article26. Smeds A, Hemmann K, Jakava-Viljanen M, Pelkonen S, Imberechts H, Palva A. Characterization of the adhesin of Escherichia coli F18 fimbriae. Infect Immun. 2001; 69:7941–7945.
Article27. da Silva AS, Valadares GF, Penatti MPA, Brito BG, da Silva Leite D. Escherichia coli strains from edema disease: O serogroups, and genes for Shiga toxin, enterotoxins, and F18 fimbriae. Vet Microbiol. 2001; 80:227–233.28. Streatfield SJ, Jilka JM, Hood EE, Turner DD, Bailey MR, Mayor JM, Woodard SL, Beifuss KK, Horn ME, Delaney DE, Tizard IR, Howard JA. Plant-based vaccines: unique advantages. Vaccine. 2001; 19:2742–2748.
Article29. Tiels P, Verdonck F, Coddens A, Ameloot P, Goddeeris B, Cox E. Monoclonal antibodies reveal a weak interaction between the F18 fimbrial adhesin FedF and the major subunit FedA. Vet Microbiol. 2007; 119:115–120.
Article30. Tiels P, Verdonck F, Coddens A, Goddeeris B, Cox E. The excretion of F18+ E. coli is reduced after oral immunisation of pigs with a FedF and F4 fimbriae conjugate. Vaccine. 2008; 26:2154–2163.
Article31. Walmsley AM, Arntzen CJ. Plants for delivery of edible vaccines. Curr Opin Biotechnol. 2000; 11:126–129.
Article32. Wang L, Liu B, Kong Q, Steinrück H, Krause G, Beutin L, Feng L. Molecular markers for detection of pathogenic Escherichia coli strains belonging to serogroups O138 and O139. Vet Microbiol. 2005; 111:181–190.
Article33. Weiner HL, van Rees EP. Mucosal tolerance. Immunol Lett. 1999; 69:3–4.
Article34. Yahong H, Liang W, Pan A, Zhou Z, Wang Q, Huang C, Chen J, Zhang D. Protective immune response of bacterially-derived recombinant FaeG in piglets. J Microbiol. 2006; 44:548–555.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunostimulating and Prophylactic Effect of Escherichia coli Extract in a Mouse Model of Lipopolysaccharide-induced Cystitis
- Expression of P, type 1 fimbriae, HEp-2 cell adherence, and colonization factor antigens of uropathogenic escherichia coli
- Evaluation of systemic and mucosal immune responses in mice administered with novel recombinant Salmonella vaccines for avian pathogenic Esherichia coli
- Expression of human CTL?4 extracellular domain in escherichia coli
- Escherichia coli O157: H7 Infection