Immune Netw.
2013 Oct;13(5):199-204. 10.4110/in.2013.13.5.199.
Syntenin Is Expressed in Human Follicular Dendritic Cells and Involved in the Activation of Focal Adhesion Kinase
- Affiliations
-
- 1Department of Microbiology and Immunology, Kangwon National University School of Medicine, Chuncheon 200-701, Korea. jchoe@kangwon.ac.kr
- 2Department of Biochemistry, College of Natural Sciences, Kangwon National University, Chuncheon 200-701, Korea.
- 3Department of Food Nutritional Sciences, Hanbuk University, Dongducheon 483-777, Korea.
- KMID: 1493614
- DOI: http://doi.org/10.4110/in.2013.13.5.199
Abstract
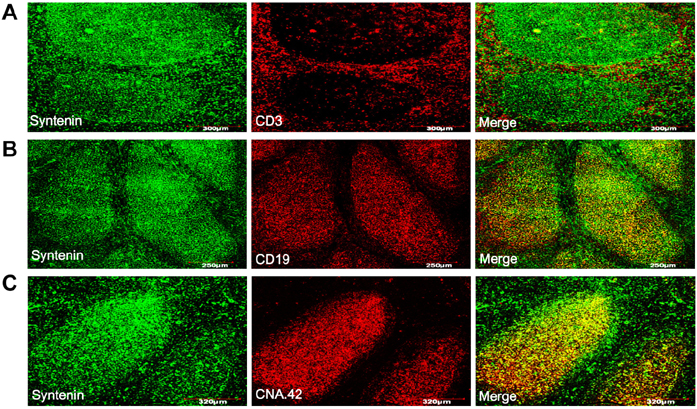
- Syntenin is an adaptor molecule containing 2 PDZ domains which mediate molecular interactions with diverse integral or cytoplasmic proteins. Most of the results on the biological function of syntenin were obtained from studies with malignant cells, necessitating exploration into the role of syntenin in normal cells. To understand its role in normal cells, we investigated expression and function of syntenin in human lymphoid tissue and cells in situ and in vitro. Syntenin expression was denser in the germinal center than in the extrafollicular area. Inside the germinal center, syntenin expression was obvious in follicular dendritic cells (FDCs). Flow cytometric analysis with isolated cells confirmed a weak expression of syntenin in T and B cells and a strong expression in FDCs. In FDC-like cells, HK cells, most syntenin proteins were found in the cytoplasm compared to weak expression in the nucleus. To study the function of syntenin in FDC, we examined its role in the focal adhesion of HK cells by depleting syntenin by siRNA technology. Knockdown of syntenin markedly impaired focal adhesion kinase phosphorylation in HK cells. These results suggest that syntenin may play an important role in normal physiology as well as in cancer pathology.
Keyword
MeSH Terms
Figure
Reference
-
1. Grootjans JJ, Zimmermann P, Reekmans G, Smets A, Degeest G, Dürr J, David G. Syntenin, a PDZ protein that binds syndecan cytoplasmic domains. Proc Natl Acad Sci USA. 1997; 94:13683–13688.2. Beekman JM, Coffer PJ. The ins and outs of syntenin, a multifunctional intracellular adaptor protein. J Cell Sci. 2008; 121:1349–1355.
Article3. Gimferrer I, Ibanez A, Farnos M, Sarrias M-R, Funutria R, Rosello S, Zimmermann P, David G, Vives J, Serra-Pages C, Lozano F. The lymphocyte receptor CD6 interacts with syntenin-1, a scaffolding protein containing PDZ domains. J Immunol. 2005; 175:1406–1414.
Article4. Latysheva N, Muratov G, Rajesh S, Padgett M, Hotchin NA, Overduin M, Berditchevski F. Syntenin-1 is a new component of tetraspanin-enriched microdomains: mechanisms and consequences of the interaction of syntenin-1 with CD63. Mol Cell Biol. 2006; 26:7707–7718.
Article5. Chen F, Du Y, Zhang Z, Chen G, Zhang M, Shu H-B, Zhai Z, Chen D. Syntenin negatively regulates TRAF6-mediated IL-1R/TLR4 signaling. Cell Signal. 2008; 20:666–674.
Article6. Koo TH, Lee JJ, Kim EM, Kim KW, Kim HD, Lee JH. Syntenin is overexpressed and promotes cell migration in metastatic human breast and gastric cancer cell lines. Oncogene. 2002; 21:4080–4088.
Article7. Gangemi R, Mirisola V, Barisione G, Fabbi M, Brizzolara A, Lanza F, Mosci C, Salvi S, Gualco M, Truini M, Angelini G, Boccardo S, Cilli M, Airoldi I, Queirolo P, Jager MJ, Daga A, Pfeffer U, Ferrini S. Mda-9/Syntenin Is expressed in uveal melanoma and correlates with metastatic progression. PLoS One. 2012; 7:e29989.
Article8. Boukerche H, Su ZZ, Prevot C, Sarkar D, Fisher PB. mda-9/Syntenin promotes metastasis in human melanoma cells by activating c-Src. Proc Natl Acad Sci USA. 2008; 105:15914–15919.
Article9. Choe J, Kim HS, Armitage J, Choi YS. The functional role of BCR stimulation and IL-4 in the generation of human memory B cells from germinal center B cells. J Immunol. 1997; 159:3757–3766.10. Kim HS, Zhang X, Choi YS. Activation and proliferation of follicular dendritic cell-like cells by activated T lymphocytes. J Immunol. 1994; 153:2951–2961.11. Kim J, Lee S, Kim YM, Jeoung DI, Choe J. Human follicular dendritic cells promote germinal center B cell survival by providing prostaglandins. Mol Immunol. 2013; 55:418–423.
Article12. Cho W, Hong SH, Choe J. IL-4 and HDAC inhibitors suppress cyclooxygenase-2 expression in human follicular dendritic cells. Immune Netw. 2013; 13:75–79.
Article13. Clark EA, Grabstein KH, Shu GL. Cultured human follicular dendritic cells: Growth characteristics and interactions with B lymphocytes. J Immunol. 1992; 148:3327–3335.14. Hwangbo C, Kim J, Lee JJ, Lee JH. Activation of the integrin effector kinase focal adhesion kinase in cancer cells is regulated by crosstalk between protein kinase C and the PDZ adapter protein mda-9/syntenin. Cancer Res. 2010; 70:1645–1655.
Article15. Munoz-Fernandez R, Blanco FJ, Frecha C, Martin F, Kimatrai M, Abadia-Molina AC, Carcia-Pacheco JM, Olivares EG. Follicular dendritic cells are related to bone marrow stromal cell progenitors and to myofibroblasts. J Immunol. 2006; 177:280–289.
Article16. Lindhout E, de Groot C. Follicular dendritic cells and apoptosis: life and death in the germinal centre. Histochem J. 1995; 27:167–183.
Article17. Franchini KG. Focal adhesion kinase - The basis of local hypertrophic signaling domains. J Mol Cell Cardiol. 2012; 52:485–492.
Article18. Stehbens S, Wittmann T. Targeting and transport: How microtubules control focal adhesion dynamics. J Cell Biol. 2012; 198:481–489.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Syntenin increases the invasiveness of small cell lung cancer cells by activating p38, AKT, focal adhesion kinase and SP1
- Signal Transduction of Ras-like GTPase Activation in the Nervous System
- Increased Expression of Focal Adhesion Kinase in Thyroid Cancer: Immunohistochemical Study
- Culture of Tonsillar Follicular Dendritic Cells
- SPARC Expression in Thyroid Follicular Adenomas and Carcinomas