Korean J Physiol Pharmacol.
2008 Apr;12(2):65-71. 10.4196/kjpp.2008.12.2.65.
Glycyrrhizin Attenuates MPTP Neurotoxicity in Mouse and MPP+-Induced Cell Death in PC12 Cells
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, Chung-Ang University, Seoul 156-756, Korea. leecs@cau.ac.kr
- KMID: 1486145
- DOI: http://doi.org/10.4196/kjpp.2008.12.2.65
Abstract
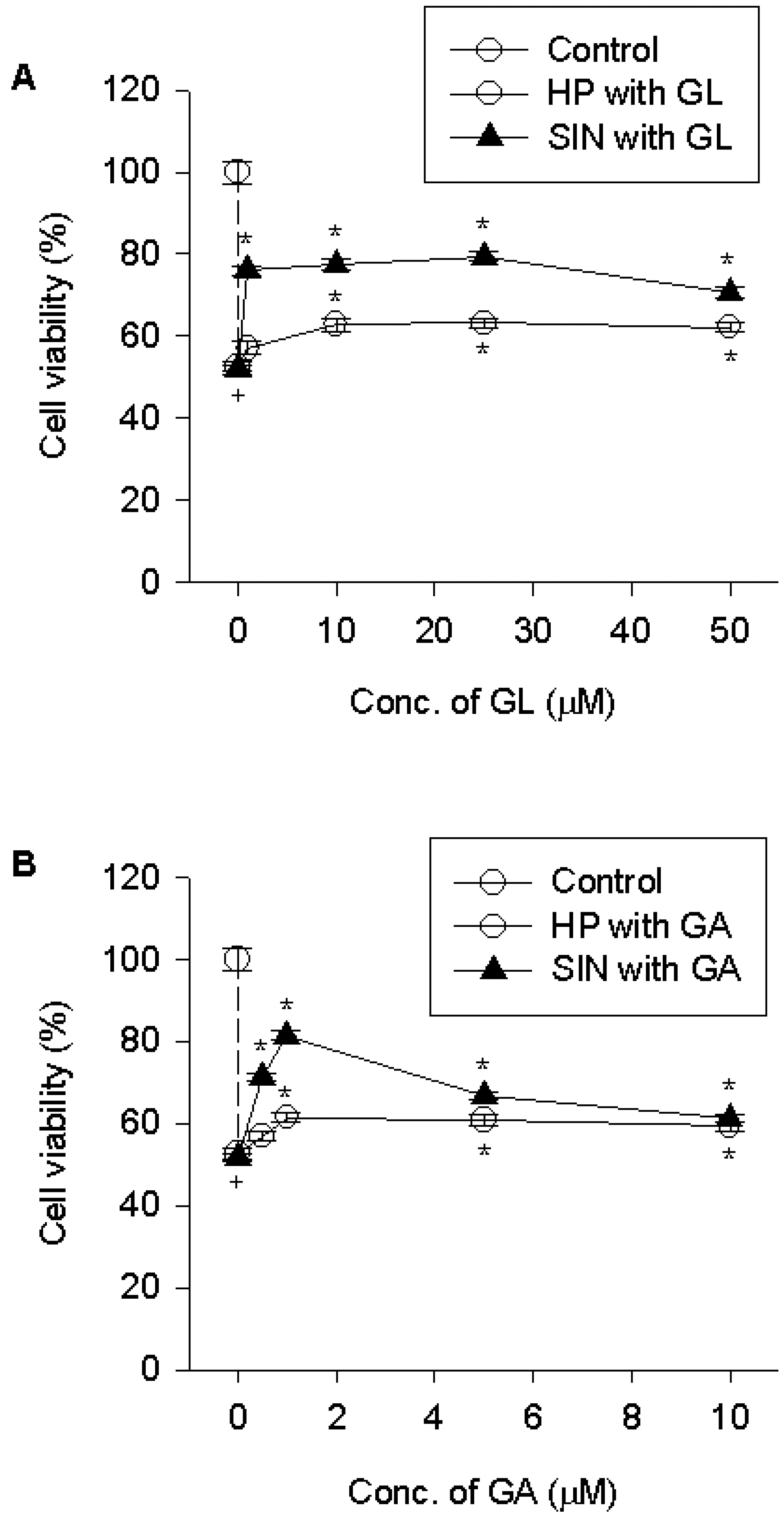
- The present study examined the inhibitory effect of licorice compounds glycyrrhizin and a metabolite 18 beta-lycyrrhetinic acid on the neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in the mouse and on the 1-methyl-4-phenylpyridinium (MPP+-induced cell death in differentiated PC12 cells. MPTP treatment increased the activities of total superoxide dismutase, catalase and glutathione peroxidase and the levels of malondialdehyde and carbonyls in the brain compared to control mouse brain. Co-administration of glycyrrhizin (16.8 mg/kg) attenuated the MPTP effect on the enzyme activities and formation of tissue peroxidation products. In vitro assay, licorice compounds attenuated the MPP+induced cell death and caspase-3 activation in PC12 cells. Glycyrrhizin up to 100 micrometer significantly attenuated the toxicity of MPP+ Meanwhile, 18beta-lycyrrhetinic acid showed a maximum inhibitory effect at 10 micrometer; beyond this concentration the inhibitory effect declined. Glycyrrhizin and 18beta-lycyrrhetinic acid attenuated the hydrogen peroxide- or nitrogen species-induced cell death. Results from this study indicate that glycyrrhizin may attenuate brain tissue damage in mice treated with MPTP through inhibitory effect on oxidative tissue damage. Glycyrrhizin and 18 beta-lycyrrhetinic acid may reduce the MPP+toxicity in PC12 cells by suppressing caspase-3 activation. The effect seems to be ascribed to the antioxidant effect.
Keyword
MeSH Terms
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
1-Methyl-4-phenylpyridinium
Animals
Antioxidants
Brain
Caspase 3
Catalase
Cell Death
Glutathione Peroxidase
Glycyrrhiza
Glycyrrhizic Acid
Hydrogen
Malondialdehyde
Mice
Nitrogen
PC12 Cells
Superoxide Dismutase
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
1-Methyl-4-phenylpyridinium
Antioxidants
Caspase 3
Catalase
Glutathione Peroxidase
Glycyrrhizic Acid
Hydrogen
Malondialdehyde
Nitrogen
Superoxide Dismutase
Figure
Reference
-
Aebi H. Catalase in vitro. Methods Enzymol. 105:121–126. 1984.Agarwal MK., Iqbal M., Athar M. Inhibitory effect of 18β-glycyrrhetinic acid on 12-O-tetradecanoyl phorbol-13-acetate- induced cutaneous oxidative stress and tumor promotion in mice. Redox Rep. 10:151–157. 2005.Bonuccelli U., Del Dotto P. New pharmacological horizons in the treatment of Parkinson disease. Neurology. 67(7 Suppl. 2):S30–S38. 2006.Cassarino DS., Fall CP., Swerdlow RH., Smith TS., Halvorsen EM., Miller SW., Parks JP., Parker Jr WD., Bennet Jr JP. Elevated reactive oxygen species and antioxidant enzyme activities in animal and cellular models of Parkinson's disease. Biochim Biophys Acta. 1362:77–80. 1997.
ArticleCassarino DS., Parks JK., Parker WD Jr., Bennett JP Jr. The Parkinsonian neurotoxin MPP+ opens the mitochondrial permeability transition pore and releases cytochrome c in isolated mitochondria via an oxidative mechanism. Biochim Biophys Acta. 1453:49–62. 1999.Fleury C., Mignotte B., Vayssière J-L. Mitochondrial reactive oxygen species in cell death signaling. Biochimie. 84:131–141. 2002.
ArticleFlohe L., Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol. 105:114–121. 1984.Gutteridge JMC., Rowley DA., Halliwell B. Superoxide dependent formation of hydroxyl radicals and lipid peroxidation in the presence of iron salts. Detection of ‘catalytic’ iron and antioxidant activity in extracellular fluids. Biochem J. 206:605–609. 1982.Hung HC., Lee EHY. MPTP produces differential oxidative stress and antioxidative responses in the nigrostriatal and mesolimbic dopaminergic pathways. Free Radic Biol Med. 24:76–84. 1998.
ArticleIshiwata S., Nakashita K., Ozawa Y., Niizeki M., Nozaki S., Tomioka Y., Mizugaki M. Fas-mediated apoptosis is enhanced by glycyrrhizin without alteration of caspase-3-like activity. Biol Pharm Bull. 22:1163–1166. 1999.
ArticleJenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 53(suppl 3):S26–S38. 2003.
ArticleJeong HG., You HJ., Park SJ., Moon AR., Chung YC., Kang SK., Chun HK. Hepatoprotective effects of 18β-glycyrrhetinic acid on carbon tetrachloride-induced liver injury: inhibition of cytochrome P450 2E1 expression. Pharmacol Res. 46:221–227. 2002.
ArticleKadota T., Yamaai T., Saito Y., Akita Y., Kawashima S., Moroi K., Inagaki N., Kadota K. Expression of dopamine transporter at the tips of growing neurites of PC12 cells. J Histochem Cytochem. 44:989–996. 1996.
ArticleKim R., Emi M., Tanabe K. Role of mitochondria as the gardens of cell death. Cancer Chemother Pharmacol. 57:545–553. 2006.
ArticleKinjo J., Hirakawa T., Tsuchihashi R., Nagao T., Okawa M., Nohara T., Okabe H. Hepatoprotective constituents in plants. 14. Effects of soyasapogenol B, sophoradiol, and their glucuronides on the cytotoxicity of tert-butyl hydroperoxide to HepG2 cells. Biol Pharm Bull. 26:1357–1360. 2003.Lee CS., Kim YJ., Ko HH., Han ES. Modulation of 1-methyl-4-phenylpyridinium-induced mitochondrial dysfunction and cell death by KATP channel block. J Neural Transm. 114:297–305. 2007.Lee DH., Han YS., Han ES., Bang H., Lee CS. Differential involvement of intracellular Ca2+ in 1-methyl-4-phenylpyridinium- or 6-hydroxydopamine-induced cell viability loss in PC12 cells. Neurochem Res. 31:851–860. 2006.Levine RL., Garland D., Oliver CN., Amici A., Climent I., Lenz A-G., Ahn B-W., Shaltiel S., Stadman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 186:464–478. 1990.Makino T., Tsubouchi R., Murakami K., Haneda M., Yoshino M. Generation of reactive oxygen species and induction of apoptosis of HL60 cells by ingredients of traditional herbal medicine, Sho-saiko-to. Basic Clin Pharmacol Toxicol. 98:401–415. 2006.
ArticleMatsui S., Matsumoto H., Sonoda Y., Ando K., Aizu-Yokota E., Sato T., Kasahara T. Glycyrrhizin and related compounds down-regulate production of inflammatory chemokines IL-8 and eotaxin 1 in a human lung fibroblast cell line. Int Immunopharmacol. 15:1633–1644. 2004.
ArticleMcCord JM., Fridovich I. Superoxide dismutase. Enzymatic function for erythrocuprein (hemocuprein). J Biol Chem. 244:6049–6055. 1969.Mignotte B., Vayssière JL. Mitochondria and apoptosis. Eur J Biochem. 252:1–15. 1998.
ArticleMosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 65:55–63. 1983.
ArticleMuijsers RBR., Folkerts G., Henricks PA., Sadeghi-Hashjin G., Nijkamp FP. Peroxynitrite: a two faced metabolite of nitric oxide. Life Sci. 60:1833–1845. 1997.Nagai T., Egashira T., Yamanaka Y., Kohno M. The protective effect of glycyrrhizin against injury of the liver caused by ischemia-reperfusion. Arch Environ Contam Toxicol. 20:432–436. 1991.
ArticleOlanow CW., Tatton WG. Etiology and pathogenesis of Parkinson's disease. Annu Rev Neurosci. 22:123–144. 1999.
ArticlePrzedborski S., Jackson-Lewis V. Mechanism of MPTP toxicity. Mov Disord. 13:35–38. 1998.Schulz JB., Matthews RT., Klockgether T., Dichgans J., Beal MF. The role of mitochondrial dysfunction and neuronal nitric oxide in animal models of neurodegenerative diseases. Mol Cell Biochem. 174:193–197. 1997.
ArticleShibata S. A drug over the millennia: pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi. 120:849–862. 2000.Tatton WG., Chalmers-Redman RME., Ju WJH., Mammen M., Carlile GW., Pong AW., Tatton NA. Propargylamines induce antiapoptotic new protein synthesis in serum-and nerve growth factor (NGF)-withdrawn, NGF-differentiated PC-12 cells. J Pharmacol Exp Ther. 301:753–764. 2002.Yokozawa T., Liu ZW., Chen CP. Protective effects of Glycyrrhizae radix extract and its compounds in a renal hypoxia (ischemia)-reoxygenation (reperfusion) model. Phytomedicine. 6:439–445. 2000.Yoshikawa M., Toyohara M., Ueda S., Shirol A., Takeuchi H., Nishiyama T., Yamada T., Fukui H., Ishizaka S. Glycyrrhizin inhibits TNF-induced, but not Fas-mediated, apoptosis in the human hepatoblastoma line HepG2. Biol Pharm Bull. 22:951–955. 1999.
ArticleZheng QZ., Lou YJ. Pathologic characteristics of immunologic injury in primary cultured rat hepatocytes and protective effect of glycyrrhizin in vitro. Acta Pharmacol Sin. 24:771–777. 2003.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential Inhibition of MPP+- or 6-Hydroxydopamine-induced Cell Viability Loss in PC12 Cells by Trifluoperazine and W-7
- Promoting Effect of Hydrogen Peroxide on 1-Methyl-4-phenylpyridinium-induced Mitochondrial Dysfunction and Cell Death in PC12 Cells
- Inhibition of Oxidative Tissue Damage and Mitochondrial Dysfunction by Glycyrrhizin in the 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine Mouse Model of Parkinson's Disease
- Role of Casein Kinase 2 in Parkinsonian Toxin 1-Methyl-4-phenylpyridinium-induced Cell Death
- Inhibitory Effect of 3,4,5-Tricaffeoylquinic Acid on Parkinsonian Toxin 1-Methyl-4-phenylpyridinium-induced Apoptosis