Korean J Physiol Pharmacol.
2008 Oct;12(5):217-223. 10.4196/kjpp.2008.12.5.217.
Role of Glucocorticoids in Fasting-induced Changes in Hypothalamic and Pituitary Components of the Growth Hormone (GH)-axis
- Affiliations
-
- 1Department of Pharmacology and Medical Research Center for Bioreaction to ROS and Biomedical Science Institute, Kyunghee University School of Medicine, Seoul, Korea. sjpark@khu.ac.kr
- KMID: 1486102
- DOI: http://doi.org/10.4196/kjpp.2008.12.5.217
Abstract
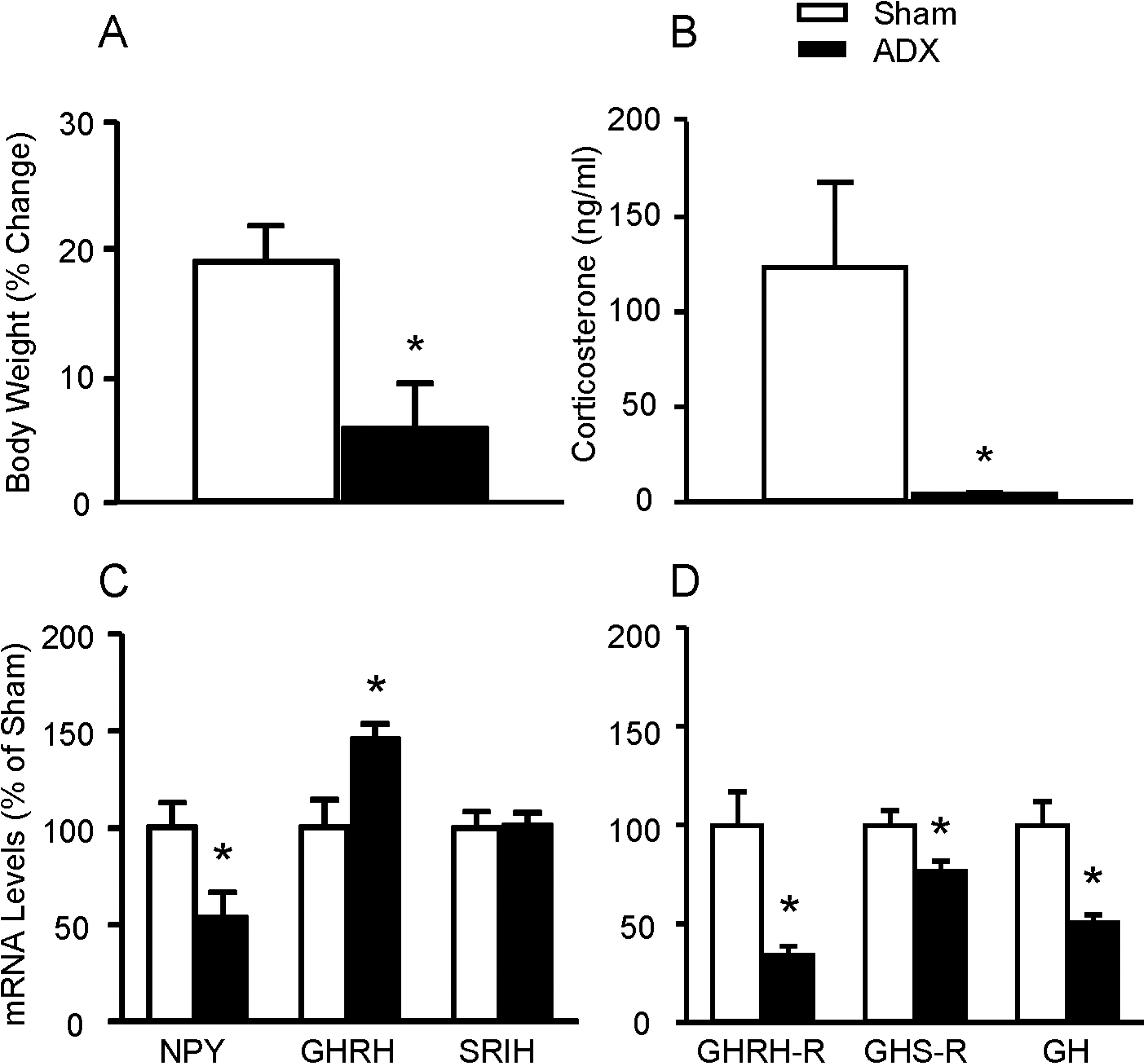
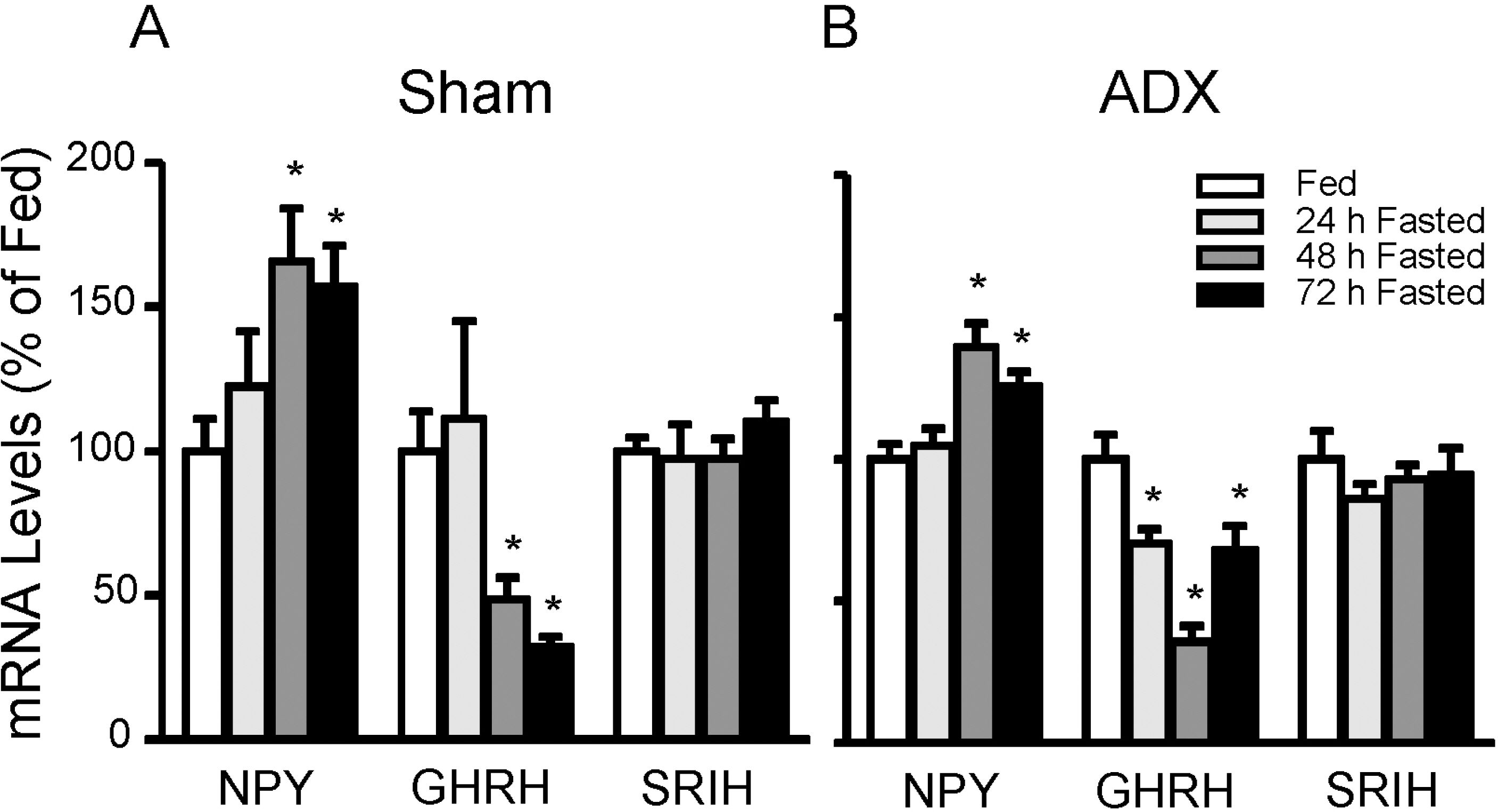
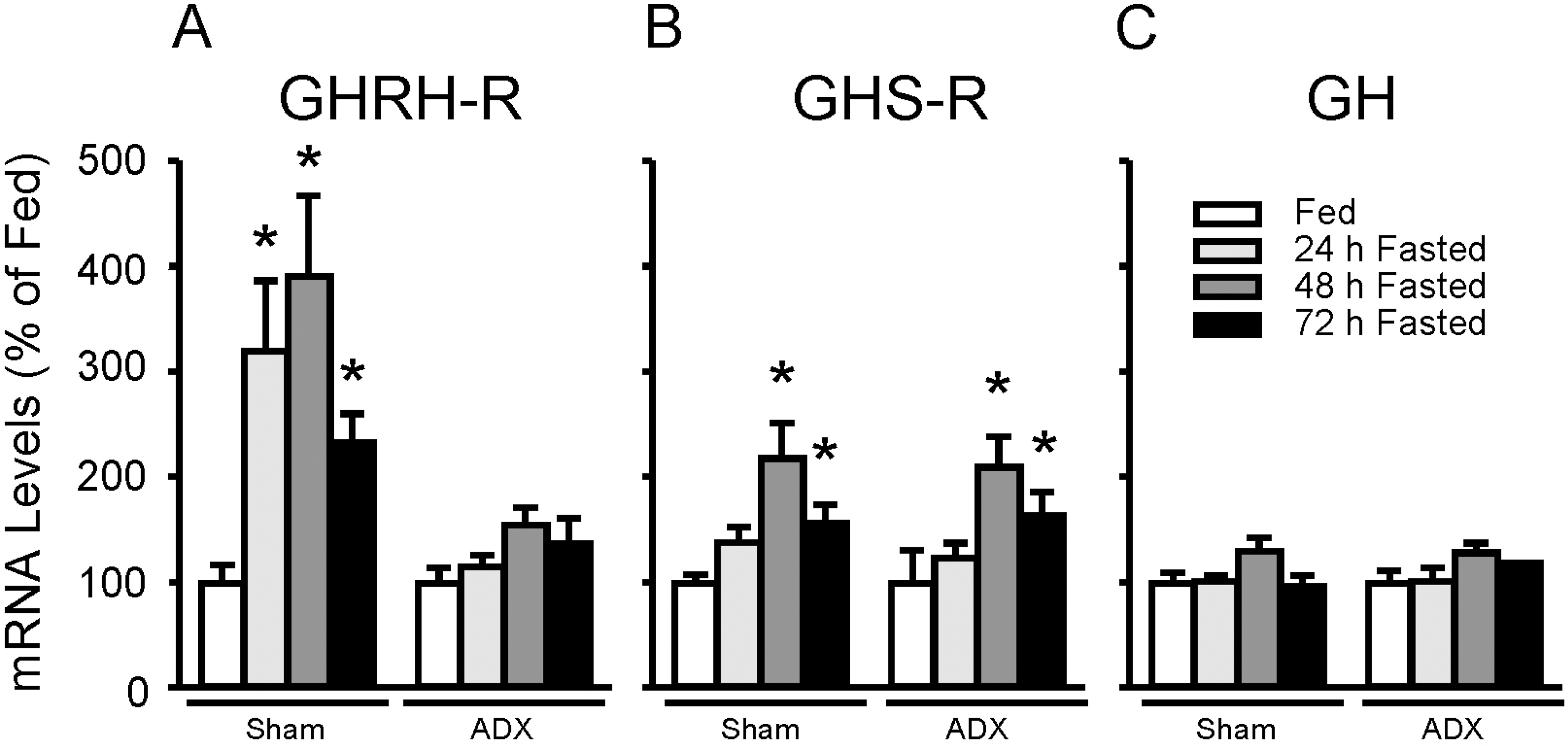
- To directly test if elevated glucocorticoids are required for fasting-induced regulation of growth hormone (GH)-releasing hormone (GHRH), GHRH receptors (GHRH-R) and ghrelin receptors (GHS-R) expression, male rats were bilaterally adrenalectomized or sham operated. After 7 days, animals were fed ad libitum or fasted for 48 h. Bilateral adrenalectomy increased hypothalamic GHRH to 146% and decreased neuropeptide Y (NPY) mRNA to 54% of SHAM controls. Pituitary GHRH-R and GHS-R mRNA levels were decreased by adrenalectomy to 30% and 80% of sham-operated controls. In sham- operated rats, fasting suppressed hypothalamic GHRH (49%) and stimulated NPY (166%) mRNA levels, while fasting increased pituitary GHRH-R (391%) and GHS-R (218%) mRNA levels. However, in adrenalectomized rats, fasting failed to alter pituitary GHRH-R mRNA levels, while the fasting-induced suppression of GHRH and elevation of NPY and GHS-R mRNA levels remained intact. In fasted adrenalectomized rats, corticosterone replacement increased GHRH-R mRNA levels and intensified the fasting-induced decrease in GHRH, but did not alter NPY or GHS-R response. These data suggest that elevated glucocorticoids mediate the effects of fasting on hypothalamic GHRH and pituitary GHRH-R expression, while glucocorticoids are likely not the major determinant in fasting-induced increases in hypothalamic NPY and pituitary GHS-R expression.
Keyword
MeSH Terms
-
Adrenalectomy
Animals
Corticosterone
Fasting
Glucocorticoids
Growth Hormone
Humans
Male
Neuropeptide Y
Rats
Receptors, Ghrelin
Receptors, Neuropeptide
Receptors, Pituitary Hormone-Regulating Hormone
RNA, Messenger
Salicylamides
Corticosterone
Glucocorticoids
Growth Hormone
Neuropeptide Y
RNA, Messenger
Receptors, Ghrelin
Receptors, Neuropeptide
Receptors, Pituitary Hormone-Regulating Hormone
Salicylamides
Figure
Cited by 1 articles
-
Direct Corticosteroid Modulation of GABAergic Neurons in the Anterior Hypothalamic Area of GAD65-eGFP Mice
Seung Yub Shin, Tae Hee Han, So Yeong Lee, Seong Kyu Han, Jin Bong Park, Ferenc Erdelyi, Gabor Szabo, Pan Dong Ryu
Korean J Physiol Pharmacol. 2011;15(3):163-169. doi: 10.4196/kjpp.2011.15.3.163.
Reference
-
Alvarez CV., Mallo F., Burguera B., Cacicedo L., Dieguez C., Casanueva FF. Evidence for a direct pituitary inhibition by free fatty acids of in vivo growth hormone responses to growth hormone- releasing hormone in the rat. Neuroendocrinology. 53:185–189. 1991.Ariyasu H., Takaya K., Tagami T., Ogawa Y., Hosoda K., Akamizu T., Suda M., Koh T., Natsui K., Toyooka S., Shirakami G., Usui T., Shimatsu A., Doi K., Hosoda H., Kojima M., Kangawa K., Nakao K. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 86:4753–4758. 2001.
ArticleBornfeldt KE., Arnqvist HJ., Enberg B., Matthews LS., Norstedt G. Regulation of insulin-like growth factor-1 and growth hormone receptor gene expression by diabetes and nutritional state in rat tissues. J Endocrinol. 122:651–656. 1989.Bruno JF., Olchovsky D., White JD., Leidy JW., Song J., Berelowitz M. Influence of food deprivation in the rat on hypothalamic expression of growth hormone-releasing factor and somatostatin. Endocrinology. 127:2111–2116. 1990.
ArticleBruno JF., Xu Y., Song J., Berelowitz M. Pituitary and hypothalamic somatostatin receptor subtype messenger ribonucleic acid expression in the food-deprived and diabetic rat. Endocrinology. 135:1787–1792. 1994.
ArticleCarro E., Senaris RM., Seoane LM., Frohman LA., Arimura A., Casaneuva FF., Dieguez C. Role of GHRH and somatostatin on leptin-induced GH secretion. Neuroendocrinology. 69:3–10. 1999.Casaneuva FF., Villanueva L., Dieguez C., Diaz Y., Cabranes JA., Szoke B., Scanlon MF., Schally AV., Fernandez-Cruz A. Free fatty acids block growth hormone (GH) releasing hormone-stimulated GH secretion in man directly at the pituitary. J Clin Endocrinol Metab. 65:634–642. 1987.Fairhall KM., Gabrielsson BG., Robinson ICAF. Effect of food withdrawal and insulin on growth hormone secretion in the guinea pig. Endocrinology. 127:716–723. 1990.
ArticleFife SK., Brogan RS., Giustina A., Wehrenberg WB. Immunocyto-chemical and molecular analysis of the effects of glucocorticoid treatment on the hypothalamic-somatotropic axis in the rat. Neuroendocrinology. 64:131–138. 1996.
ArticleGhigo MC., Torsello A., Grilli R., Luoni M., Locatelli V., Muller EE. Effects of GH and IGF-I administration on GHRH and somatostatin mRNA levels: I. A study on ad libitum fed and starved adult male rats. J Endocrinol Invest. 20:144–150. 1997.
ArticleGianotti L., Pincelli AI., Scacchi M., Rolla M., Bellitti D., Arvat E., Lanfranco F., Torsello A., Ghigo E., Cavagnini R., Muller EE. Effects of recombinant human insulin-like growh factor I administration on spontaneous and growth hormone (GH)-releasing hormone-stimulated GH secretion in anorexia nervosa. J Clin Endocrinol Metab. 85:2805–2809. 2000.Gianotti L., Rolla M., Arvat E., Belliti D., Valleto MR., Ferdeghini M., Ghigo E., Mueller EE. Effect of somatostatin infusion on the somatotrope responsiveness to growth hormone-releasing hormone in patients with anorexia nervosa. Biol Psychiat. 45:334–339. 1999.
ArticleHanson ES., Levin N., Dallman MF. Elevated corticosterone is not required for the rapid induction of neuropeptide Y expression by an overnight fast. Endocrinology. 138:1041–1047. 1997.Henricks DM., Jenkins TC., Ward JR., Krishnan CS., Grimes L. Endocrine responses and body composition changes during feed restriction and realimentation in young bulls. J Anim Sci. 72:2289–2297. 1994.Ho KY., Veldhuis JD., Johnson ML., Furlanetto R., Evans WS., Alberti KGMM., Thorner MO. Fasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in man. J Clin Invest. 81:968–975. 1988.
ArticleHo PJ., Friberg RD., Barkan AL. Regulation of pulsatile growth hormone secretion by fasting in normal subjects and patients with acromegaly. J Clin Endocrinol Metab. 75:812–819. 1992.
ArticleJanowski BA., Ling NC., Giustina A., Wehrenberg WB. Hypothalamic regulation of growth hormone secretion during food deprivation in the rat. Life Sci. 52:981–987. 1993.
ArticleKamegai J., Tamura H., Shimizu T., Ishii S., Sugihara H., Wakabayashi I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes. 50:2438–2443. 2001.
ArticleKamegai J., Unterman TG., Frohman LA., Kineman RD. Hypothalamic/pituitary-axis of the spontaneous dwarf rat: autofeedback regulation of growth hormone (GH) includes suppression of GH releasing-hormone receptor messenger ribonucleic acid. Endocrinology. 139:3554–3560. 1998a.
ArticleKamegai J., Wakabayashi I., Miyamoto K., Unterman TG., Kineman RD., Frohman LA. Growth hormone (GH)-dependent regulation of pituitary GH secretagogue receptor (GHS-R) mRNA levels in the spontaneous dwarf rat. Neuroendocrinology. 68:312–318. 1998b.Kim E., Sohn S., Lee M., Jung J., Kineman RD., Park S. Differential responses of the growth hormone axis in two rat models of streptozotocin-induced insulinopenic diabetes. J Endocrinol. 188:263–270. 2006.
ArticleKim MS., Yoon CY., Park KH., Shin CS., Park KS., Kim SY., Cho BY., Lee HK. Changes in ghrelin and ghrelin receptor expression according to feeding status. Neuroreport. 14:1317–1320. 2003.
ArticleKorbonits M., Little JA., Forsling L., Tringali G., Costa A., Navarra P., Trainer PJ., Grossman AB. The effect of growth hormone secretagogues and neuropeptide Y on hypothalamic hormone release from acute rat hypothalamic explants. J Neuroendocrinol. 11:521–528. 1999.
ArticleLam KS., Lee MF., Tam SP., Srivastava G. Gene expression of the receptor for growth hormone-releasing hormone is physiologically regulated by glucocorticoids and estrogen. Neuroendocrinology. 63:475–480. 1996.Lam KS., Srivastava G. Gene expression of hypothalamic somatostatin and growth hormone-releasing hormone in dexamethasone-treated rats. Neuroendocrinology. 66:2–8. 1997.
ArticleMaccario M., Procopio M., Loche S., Cappa M., Martina V., Camanni F., Ghigo E. Interaction of free fatty acids and arginine on growth hormone secretion in man. Metabolism. 43:223–226. 1994.
ArticleMiller TL., Mayo KE. Glucocorticoids regulate pituitary growth hormone-releasing hormone receptor messenger ribonucleic acid expression. Endocrinology. 138:2458–2465. 1997.
ArticleMinami S., Kamegai J., Sugihara H., Suzuki N., Wakabayashi I. Growth hormone inhibits its own secretion by acting on the hypothalamus through its receptors on neuropeptide Y neurons in the arcuate nucleus and somatostatin neurons in the periventricular nucleus. Endocr J. 48(Suppl):19–26. 1998.
ArticleObici S., Feng Z., Morgan K., Stein D., Karkanias G., Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes. 51:271–275. 2002.
ArticleOhyama T., Sato M., Ohye H., Murao K., Niimi M., Takahara J. Effects of adrenalectomy and glucocorticoid receptor antagonist, RU38486, on pituitary growth hromone-releasing hormone receptor gene expression in rats. Peptides. 19:1063–1067. 1998.Park S., Sohn S., Kineman RD. Fasting-induced changes in the hypothalamic-pituitary-GH axis in the absence of GH expression: lessons from the spontaneous dwarf rat. J Endocrinol. 180:369–378. 2004.
ArticleRiedel M., Hoeft B., Blum WF., von zur Muhlen A., Brabant G. Pulsatile growth hormone secretion in normal-weight and obese men: differential metabolic regulation during energy restriction. Metabolism. 44:605–610. 1995.
ArticleSenaris RM., Lago F., Coya R., Pineda J., Dieguez C. Regulation of hypothalamic somatostatin, growth hormone- releasing hormone, and growth hormone receptor messenger ribonucleic acid by glucocorticoids. Endocrinology. 137:5236–5241. 1996.Shintani M., Ogawa Y., Ebihara K., Aizawa-Abe M., Miyanaga F., Takaya K., Hayashi T., Inoue G., Hosoda K., Kojima M., Kangawa K., Nakao K. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. 50:227–232. 2001.
ArticleStoving RK., Veldhuis JD., Flyvbjerg A., Vinten J., Hangaard J., Koldkjaer OG., Kristiansen J., Hagen C. Jointly amplified basal and pulasatile growth hormone (GH) secretion and increased process irregularity in women with anorexia nervosa: indirect evidence for disruption of feedback regulation within the GH-Insulin-like growth factor 1 axis. J Clin Endocrinol Metab. 84:2056–2063. 1999.Sugihara H., Emoto N., Shibasaki T., Minami S., Wakabayashi I. Increased pituitary growth hormone-releasing factor (GRF) receptor messenger ribonucleic acid expression in food-deprived rats. Brain Res. 742:355–358. 1996.
ArticleTamura H., Kamegai J., Sugihara H., Kineman RD., Frohman LA., Wakabayashi I. Glucocorticoids regulate pituitary growth hormone secretagogue receptor gene expression. J Neuroendocrinol. 12:481–485. 2000.
ArticleTannenbaum GS., Rorstad O., Brazeau P. Effects of prolonged food deprivation on the ultradian growth hormone rhythm and immunoreactive somatostatin tissue levels in the rat. Endocrinology. 104:1733–1738. 1979.
ArticleThomas GB., Bennett PA., Carmignac DF., Robinson ICAF. Glucocorticoid regulation of growth hormone (GH) secretagogue-induced growth responses and GH secretagogue receptor expression in the rat. Growth Horm IGF Res. 10:45–52. 2000.
ArticleThomas GB., Mercer JE., Karalis T., Rao A., Cummins JT., Clarke IJ. Effect of restricted feeding on the concentrations of growth hormone (GH), gonadotropins, and prolactin (PRL) in plasma, and on the amounts of messenger ribonucleic acid for GH, gonadotropin subunits, and PRL in the pituitary glands of adult ovariectomized ewes. Endocrinology. 126:1361–1367. 1990.
ArticleToshinai K., Mondal MS., Nakazato M., Date Y., Murakami N., Kojima M., Kangawa K., Matsukura S. Upregulation of Ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun. 281:1220–1225. 2001a.
ArticleToshinai K., Mondal SS., Nakazato M., Date Y., Murakami N., Kojima M., Kangawa K., Matsukura S. Upregulation of ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun. 281:1220–1225. 2001b.
ArticleVuagnat BA., Pierroz DD., Lalaoui M., Englaro P., Pralong FP., Blum WF., Aubert ML. Evidence for a leptin-neuropeptide Y axis for the regulation of growth hormone secretion in the rat. Neuroendocrinology. 67:291–300. 1998.
ArticleWilkinson CW., Engeland WC., Shinsako J., Dallman MF. Nonsteroidal adrenal feedback demarcates two types of pathways to CRF-ACTH release. Am J Physiol. 240:E136–E145. 1981.
ArticleWillesen MG., Kirstensen P., Romer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 70:306–316. 1999.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuroendocrine Regulation of Growth Hormone Secretion
- Hypothalamic-Pituitary-Adrenal Axis and Epilepsy
- Changes in Hypothalamic-pituitary-growth Hormone (GH) Axis by Fasting: Study on the Differences between Male and Female Rats
- Study on the Reciprocal Interactions between Growth Hormone and Ghrelin in the Rat
- Growth Hormone Treatment in Children with Chronic Kidney Disease