J Bacteriol Virol.
2008 Sep;38(3):127-137. 10.4167/jbv.2008.38.3.127.
Amantadine and Zanamivir Resistance of Influenza A/H3N2 Viruses Isolated in Korea, 2002/03~2003/04
- Affiliations
-
- 1Division of Influenza and Respiratory Viruses, National Institute of Health, Korea Center for Disease Control and Prevention, Seoul, Korea. ckang@nih.go.kr
- 2Department of Life Sciences, School of Life Sciences and Biotechnology, Korea University, Seoul, Korea.
- KMID: 1483971
- DOI: http://doi.org/10.4167/jbv.2008.38.3.127
Abstract
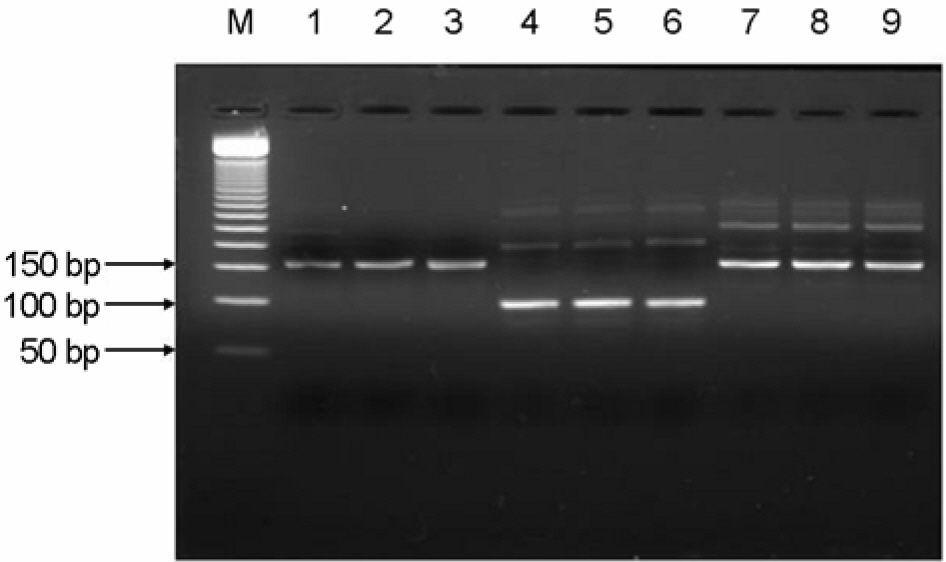
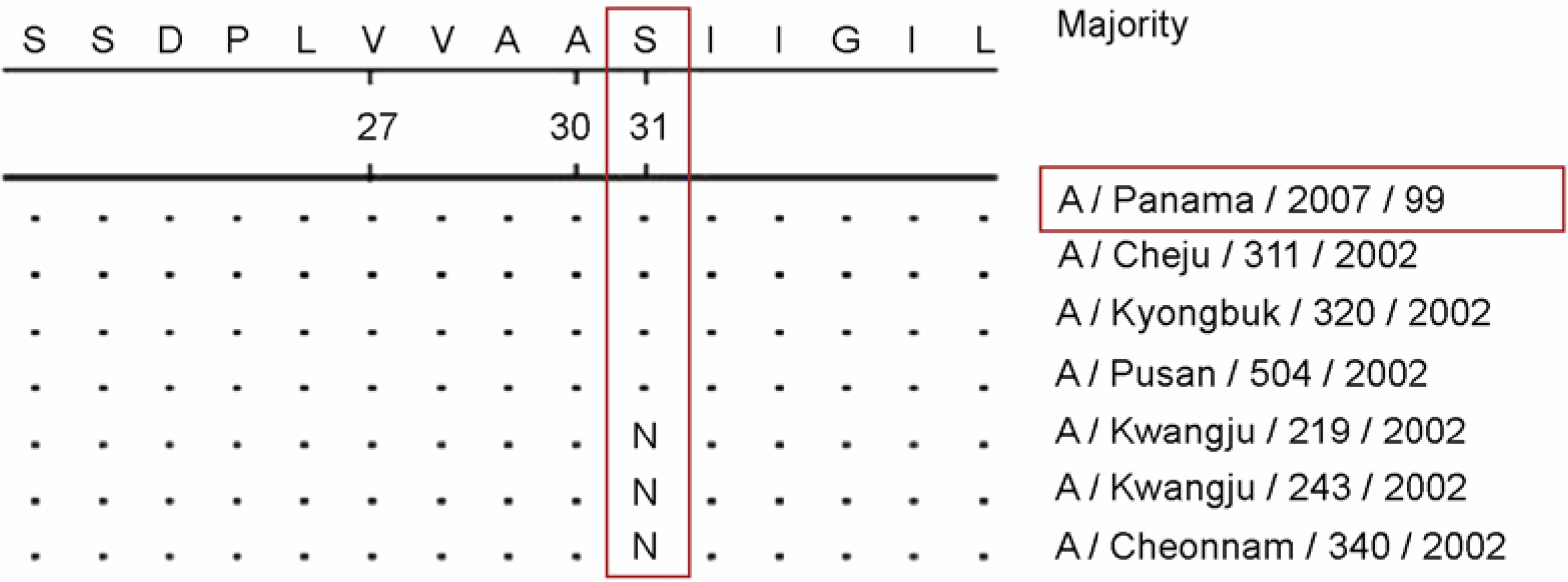
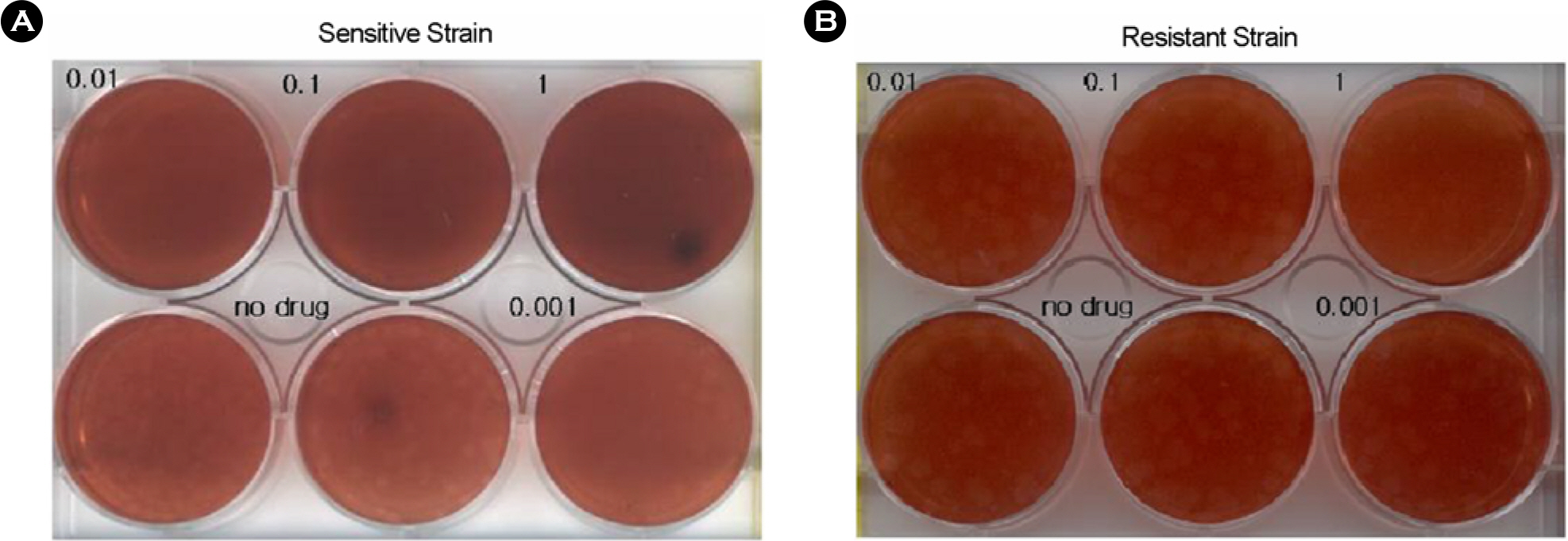
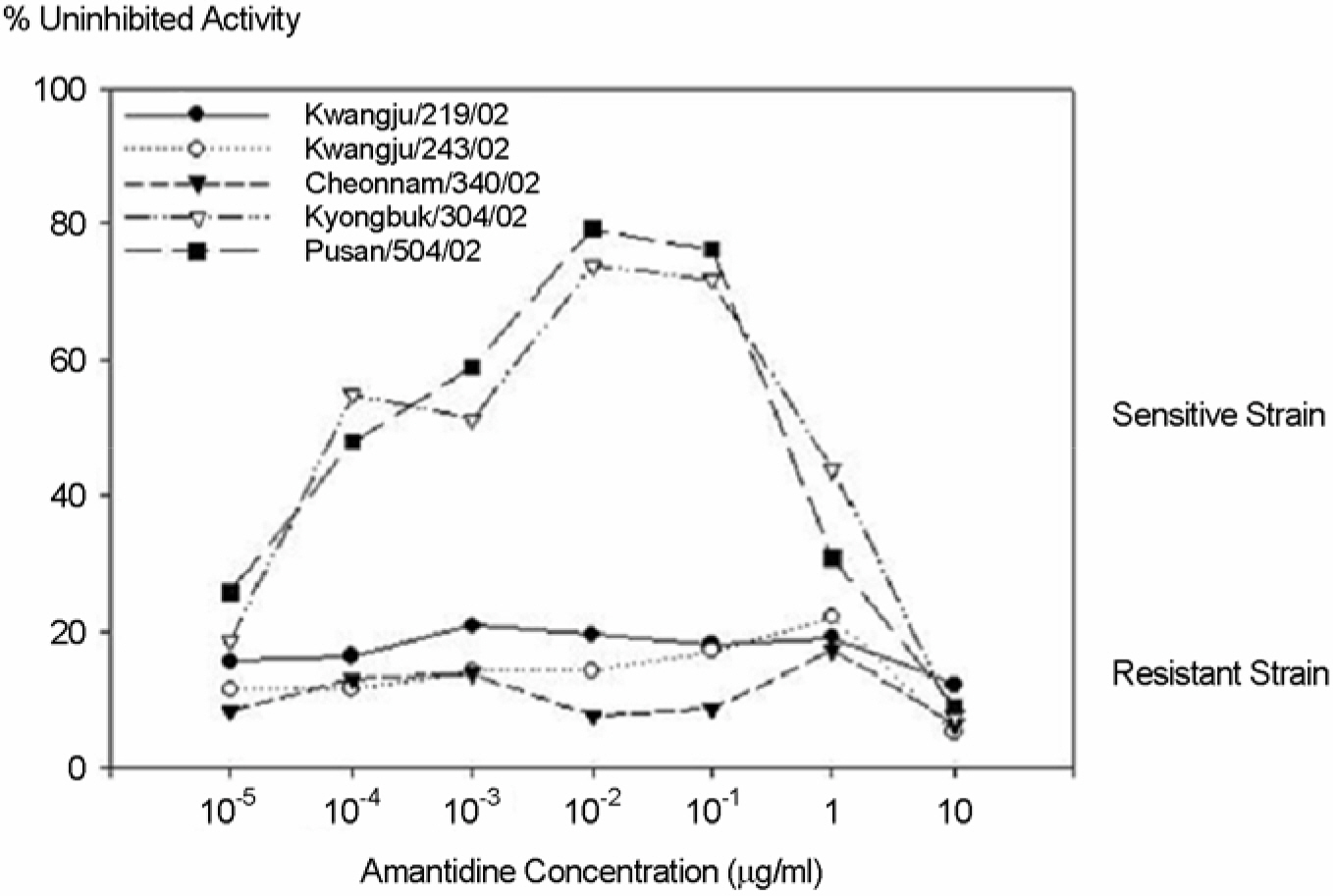
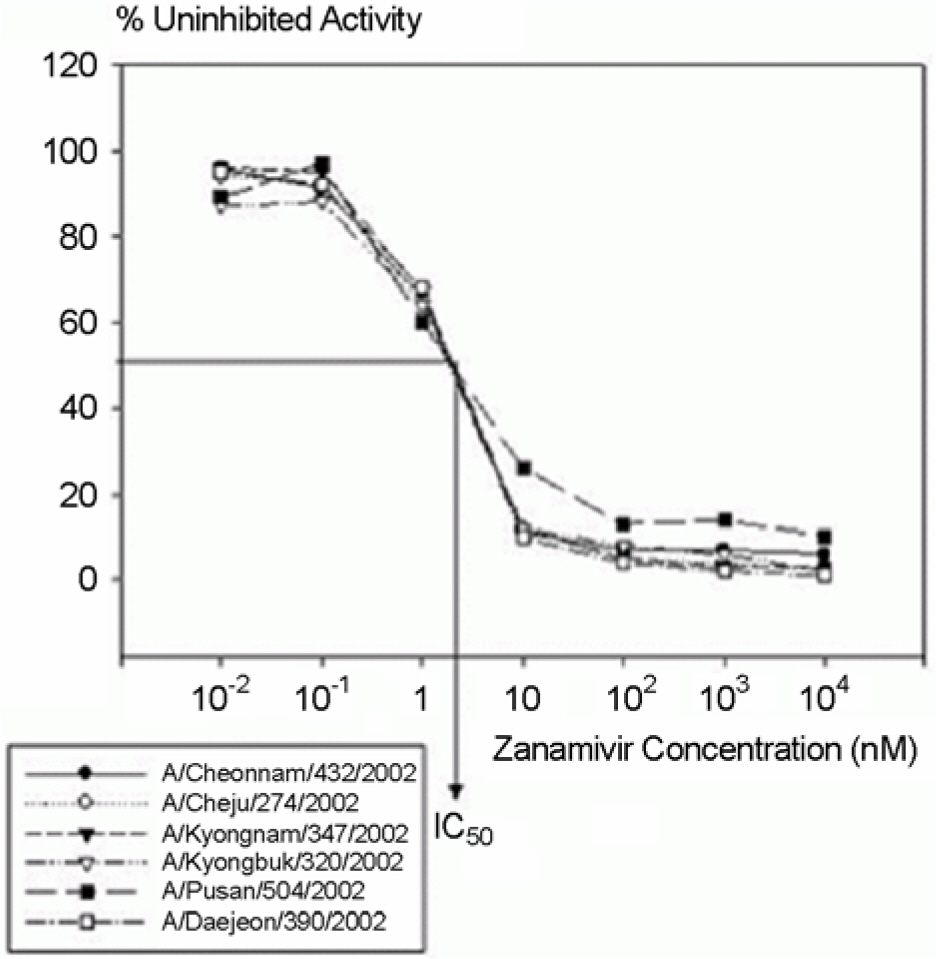
- To investigate the emergence and prevalence of antiviral resistance, we analyzed influenza A/H3N2 viruses isolated in Korea during 2002/03 to 2003/04 season by genetic and phenotypic assay. For the genetic analysis to the amantadine, an M2 protein inhibitor, the M gene was amplified by RT-PCR and regions corresponding to the amino acid at positions 27, 30, and 31 were amplified by nested PCR with size of 154 bp, 95 bp, and 153 bp fragments, respectively. A total of 3 of 31 (9.7%) viruses were found to be mutated by restriction fragment length polymorphism (RFLP) with Sca I and sequence analysis, showing the single amino acid change (Ser to Asn) at position 31. Also it was observed that their growths in Madin-Darby Canine Kidney (MDCK) cells were unaffected by amantadine (up to 1 microgram/ml) in both plaque assay and WST-1 assay, confirming that these viruses were resistant against amantadine. We also examined the resistant pattern against zanamivir, a neuraminidase inhibitor, for 15 Korean influenza A/H3N2 viruses isolated in 2002~2003 season. Sequence analysis showed that there were no genetic changes of NA genes including R292K, K274Y, R152K, and E119V which were related to resistance against the neuraminidase inhibitor. In the NA inhibition assay to zanamivir, Korean isolates were found to be sensitive, ranging from 0.17 nM to 1.77 nM in 50% inhibitory concentration (IC(50)). These results suggest that monitoring for the antiviral resistance should be intensified and maintained to provide guideline for prophylaxis and treatment of influenza in Korea.
Keyword
MeSH Terms
Figure
Reference
-
1). Bantia S., Ghate AA., Ananth SL., Babu YS., Air GM., Walsh GM. Generation and characterization of a mutant of influenza A virus selected with the neuraminidase inhibitor BCX-140. Antimicrob Agents Chemother. 42:801–807. 1998.
Article2). Belshe RB., Smith MH., Hall CB., Betts R., Hay AJ. Genetic basis of resistance to rimantadine emerging during treatment of influenza virus infection. J Virol. 62:1508–1512. 1988.
Article3). Blick TJ., Sahasrabudhe A., McDonald M., Owens IJ., Morley PJ., Fenton RJ., McKimm-Breschkin JL. The interaction of neuraminidase and hemagglutinin mutations in influenza virus in resistance to 4-Guanidino-Neu5Ac2en. Virology. 246:95–103. 1998.
Article4). Bright RA., Medina MJ., Xu X., Perez-Oronoz G., Wallis TR., Davis XM., Povinelli L., Cox NJ., Klimov AI. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: a cause for concern. Lancet. 366:1175–1181. 2005.
Article5). Gubareva LV., Bethell R., Hart GJ., Murti KG., Penn CR., Webster RG. Characterization of mutants of influenza A virus selected with the neuraminidase inhibitor 4-Guanidino-Neu5Ac2en. J Virol. 70:1818–1827. 1996.
Article6). Gubareva LV., Kaiser L., Hayden FG. Influenza virus neuraminidase inhibitors. Lancet. 355:827–835. 2000.
Article7). Hay AJ., Wolstenholme AJ., Skehel JJ., Smith MH. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 4:3021–3024. 1985.
Article8). Hungnes O. The role of genetic analysis in influenza virus surveillance and strain characterisation. Vaccine. 20:45–49. 2002.
Article9). Ilyushina NA., Govorkova EA., Russell CJ., Hoffmann E., Webster RG. Contribution of H7 haemagglutinin to amantadine resistance and infectivity of influenza virus. J Gen Virol. 88:1266–1274. 2007.
Article10). Ilyushina NA., Govorkova EA., Webster RG. Detection of amantadine-resistant variants among avian influenza viruses isolated in North America and Asia. Virology. 341:102–106. 2005.
Article11). Kim YY., Lee JY., Hwang JH., Kim KA., Jang SW., Park MS., Kim WJ., Cho HW., Lee HH., Kang C. Characterization of hemagglutinin and neuraminidase genes and oseltamivir resistance of influenza viruses isolated in Korea. J Bacteriol Virol. 35:149–155. 2005.12). Lamb RA., Zebedee SL., Richardson CD. Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell. 40:627–633. 1985.
Article13). McKimm-Breschkin J., Trivedi T., Hampson A., Hay A., Klimov A., Tashiro M., Hayden F., Zambon M. Neuraminidase sequence analysis and susceptibilities of influenza virus clinical isolates to zanamivir and oseltamivir. Antimicrob Agents Chemother. 47:2264–2272. 2003.
Article14). Monto AS. The role of antivirals in the control of influenza. Vaccine. 21:1796–1800. 2003.
Article15). Nakajima K. Influenza virus genome structure and encoded proteins. Nippon Rinsho. 55:2542–2546. 1997.16). Nobusawa E., Ishihara H., Morishita T., Sato K., Nakajima K. Change in receptor-binding specificity of recent human influenza A viruses (H3N2): A single amino acid change in hemagglutinin altered its recognition of sialyloligosaccharides. Virology. 278:587–596. 2000.
Article17). Pabbaraju K., Ho KC., Wong S., Shokoples S., Pang XL., Fonseca K., Fox JD. Adamantane resistance in circulating human influenza A viruses from Alberta, Canada (1970-2007). Antiviral Res. 79:81–86. 2008.
Article18). Pinto LH., Holsinger LJ., Lamb RA. Influenza virus M2 protein has ion channel activity. Cell. 69:517–528. 1992.
Article19). Rahman M., Bright RA., Kieke BA., Donahue JG., Greenlee RT., Vandermause M., Balish A., Foust A., Cox NJ., Klimov AI., Shay DK., Belongia EA. Adamantane-resistant influenza infection during the 2004~05 season. Emerg Infect Dis. 14:173–176. 2008.
Article20). Saito R., Li D., Suzuki Y., Sato I., Masaki H., Nishimura H., Kawashima T., Shirahige Y., Shimomura C., Asoh N., Degawa S., Ishikawa H., Sato M., Shobugawa Y., Suzuki H. High prevalence of amantadine resistance influenza A (H3N2) in six prefectures, Japan, in the 2005~2006 season. J Med Virol. 79:1569–1576. 2007.21). Saito R., Oshitani H., Masuda H., Suzuki H. Detection of amantadine-resistant influenza A virus strains in nursing homes by PCR-restriction fragment length polymorphism analysis with nasopharyngeal swabs. J Clin Microbiol. 40:84–88. 2002.
Article22). Saito R., Suzuki Y., Li D., Zaraket H., Sato I., Masaki H., Kawashima T., Hibi S., Sano Y., Shobugawa Y., Oguma T., Suzuki H. Increased incidence of adamantane-resistant influenza A(H1N1) and A(H3N2) viruses during the 2006~2007 influenza season in Japan. J Infect Dis. 197:630–632. 2007.
Article23). Shiraishi K., Mitamura K., Sakai-Tagawa Y., Goto H., Sugaya N., Kawaoka Y. High frequency of resistant viruses harboring different mutations in amantadine-treated children with influenza. J Infect Dis. 188:57–61. 2003.
Article24). Sidwell RW., Smee DF. In vitro and in vivo assay systems for study of influenza virus inhibitors. Antiviral Res. 48:1–16. 2000.
Article25). Steinhauer DA., Wharton SA., Skehel JJ., Wiley DC., Hay AJ. Amantadine selection of a mutant influenza virus containing an acid-stable hemagglutinin glycoprotein: evidence for virus-specific regulation of the pH of glycoprotein transport vesicles. Proc Natl Acad Sci USA. 88:11525–11529. 1991.
Article26). Sugrue RJ., Hay AJ. Structural characteristics of the M2 protein of influenza A viruses: evidence that it forms a tetrameric channel. Virology. 180:617–624. 1991.27). van Elden LJ., Nijhuis M., Schipper P., Schuurman R., van Loon AM. Simultaneous detection of influenza viruses A and B using real-time quantitative PCR. J Clin Microbiol. 39:196–200. 2001.
Article28). Wang MZ., Tai CY., Mendel DB. Mechanism by which mutations at his274 alter sensitivity of influenza A virus N1 neuraminidase to oseltamivir carboxylate and zanamivir. Antimicrob Agents Chemother. 46:3809–3816. 2002.
Article29). Wiely DC., Skehel TT. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Ann Rev Biochem. 56:365–394. 1987.30). Zambon M., Hayden FG. Global Neuraminidase Inhibitor Susceptibility Network: Position statement: global neuraminidase inhibitor susceptibility network. Antiviral Res. 49:147–156. 2001.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of Isolation and Subtyping of Influenza Virus in Seoul, During 1999-2003
- Prevention and Treatment of Influenza
- The Morphological Changes by the Time of Administration of Zanamivir in Rabbit Nasal Mucosa Infected with Influenza A Virus
- The Emergence of Oseltamivir-Resistant Seasonal Influenza A (H1N1) Virus in Korea During the 2008-2009 Season
- Epidemiological Analysis of Influenza by Laboratory Surveillance in Incheon, 2003/2004~2004/2005