J Bacteriol Virol.
2008 Dec;38(4):189-196. 10.4167/jbv.2008.38.4.189.
Detection of Virulence Genes of Staphyloccus aureus and Staphylococcus epidermidis Isolated from Suprapubic Urine from Infants with Fever
- Affiliations
-
- 1Department of Microbiology, School of Medicine, Ewha Womans University, Seoul, Korea. parkhk@ewha.ac.kr
- 2Deprtment of Microbiology, Graduate School of Medicine, Gachon University of Medicine and Science, Incheon, Korea.
- 3Department of Preventive Medicine, and 4Department of Pediatrics, School of Medicine, Ewha Womans University, Seoul, Korea.
- KMID: 1483959
- DOI: http://doi.org/10.4167/jbv.2008.38.4.189
Abstract
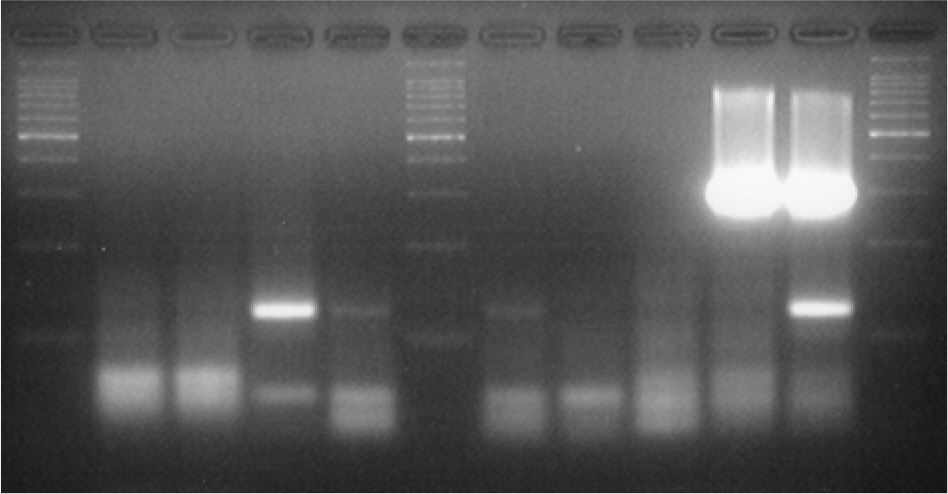
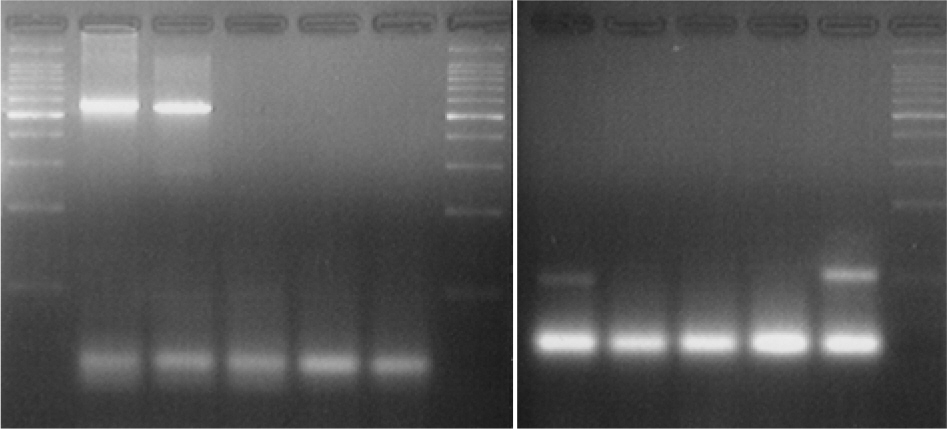
- While methicillin-resistant Staphylococcus aureus (MRSA) isolated from urinary tract infection (UTI) has recently increased in elderly adult urology patients, it has been only rarely reported in infants. Therefore, in this study to understand whether MRSA may be involved in UTI of infants who run fever without other apparent causes, we identified and counted S. aureus and S. epidermidis in suprapubic urine from 750 febrile infants via microbiological methods, and confirmed the counts via multiplex PCR. And we also detected four virulence genes, mecA, PVL, bbp and icaA genes for S. aureus and S. epidermidis via multiplex PCR in the same specimens. S. aureus (28 cases) counts were as follows: >10(4) CFU/ml (3/28), 10(2)~10(3) CFU/ml (1/28) and <10(2)~10(3) CFU/ml (24/28). S. epidermidis (26 cases) counts were as follows: >10(4) CFU/ml (2/26), 10(2)~10(3) CFU/ml (4/26) and 10(2)~10(3) CFU/ml (20/26). S. aureus virulence genes were detected in 26 cases as mecA (16/26, 59.3%), PVL (17/26, 63.0%), bbp (7/26, 26.9%) and icaA (20/26, 76.9%). S. epidermidis virulence genes were detected in 22 cases as mecA (17/22, 81.0%), PVL (15/22, 71.4%), bbp (3/22, 13.6%) and icaA (13/22, 50.1%). Therefore, mecA, PVL and icaA genes of MRSA and MRSE were detected with high positivity in urines from infants with fever. The results demonstrate that community-acquired MRSA or MRSE may be responsible for UTI incidence in febrile infants.
Keyword
MeSH Terms
Figure
Reference
-
1). Aires-de-Sousa M., Conceição T., de Lencastre H. Unusually high prevalence of nosocomial Panton-Valentine leukocidin-positive Staphylococcus aureus isolates in Cape Verde Islands. J Clin Microbiol. 44:3790–3793. 2006.2). Arciola CR., Campoccia D., Gamberini S., Donati ME., Baldassarri L., Montanaro L. Occurrence of ica genes for slime synthesis in a collection of Staphylococcus epidermidis strains from orthopedic prosthesis infections. Acta Orthop Scand. 74:617–621. 2003.3). Baba-Moussa L., Anani L., Scheftel JM., Couturier M., Riegel P., Haikou N., Hounsou F., Monteil H., Sanni A., Prévost G. Virulence factors produced by strains of Staphylococcus aureus isolated from urinary tract infections. J Hosp Infect. 68:32–38. 2008.4). Bahrain M., Vasiliades M., Wolff M., Younus F. Five cases of bacterial endocarditis after furunculosis and the ongoing saga of community-acquired methicillin-resistant Staphylococcus aureus infections. Scand J Infect Dis. 38:702–707. 2006.5). Diep BA., Sensabaugh GF., Somboona NS., Carleton HA., Perdreau-Remington F. Widespread skin and soft-tissue infections due to two methicillin-resistant Staphylococcus aureus strains harboring the genes for Panton-Valentine leucocidin. J Clin Microbiol. 42:2080–2084. 2004.6). Frebourg NB., Lefebvre S., Baert S., Lemeland JF. PCR-based assay for discrimination between invasive and contaminating Staphylococcus epidermidis strains. J Clin Microbiol. 38:877–880. 2000.7). Hoberman A., Chao HP., Keller DM., Hickey R., Davis HW., Ellis D. Prevalence of urinary tract infection in febrile infants. J Pediatr. 123:17–23. 1993.
Article8). Issartel B., Tristan A., Lechevallier S., Bruyere F., Lina G., Garin B., Lacassin F., Bes M., Vandenesch F., Etienne J. Frequent carriage of Panton-Valentine leucocidin genes by Staphylococcus aureus isolates from surgically drained abscesses. J Clin Microbiol. 43:3203–3207. 2005.9). Johnson LB., Venugopal AA., Pawlak J., Saravolatz LD. Emergence of community-associated methicillin-resistant Staphylococcus aureus infection among patients with end-stage renal disease. Infect Control Hosp Epidemiol. 27:1057–1062. 2006.10). Jones ME., Karlowsky JA., Draghi DC., Thornsberry C., Sahm DF., Bradley JS. Rates of antimicrobial resistance among common bacterial pathogens causing respiratory, blood, urine, and skin and soft tissue infections in pediatric patients. Eur J Clin Microbiol Infect Dis. 23:445–455. 2004.
Article11). Kaneko J., Kimura T., Narita S., Tomita T., Kamio Y. Complete nucleotide sequence and molecular characterization of the temperate staphylococcal bacteriophage phiPVL carrying Panton-Valentine leukocidin genes. Gene. 215:57–67. 1998.12). Karahan ZC., Tekeli A., Adaleti R., Koyuncu E., Dolapci I., Akan OA. Investigation of Panton-Valentine leukocidin genes and SCCmec types in clinical Staphylococcus aureus isolates from Turkey. Microb Drug Resist. 14:203–210. 2008.13). Lina G., Piémont Y., Godail-Gamot F., Bes M., Peter MO., Gauduchon V., Vandenesch F., Etienne J. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 29:1128–1132. 1999.14). Manzur A., Dominguez AM., Pujol M., González MP., Limon E., Hornero A., Martín R., Gudiol F., Ariza J. Community-acquired methicillin-resistant Staphylococcus aureus infections: an emerging threat in Spain. Clin Microbiol Infect. 14:377–380. 2008.15). Martineau F., Picard FJ., Lansac N., Ménard C., Roy PH., Ouellette M., Bergeron MG. Correlation between the resistance genotype determined by multiplex PCR assays and the antibiotic susceptibility patterns of Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 44:231–238. 2000.16). Moran GJ., Krishnadasan A., Gorwitz RJ., Fosheim GE., McDougal LK., Carey RB., Talan DA. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 355:666–674. 2006.17). O'Gara JP. ica and beyond: biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol Lett. 270:179–188. 2007.18). Osterlund A., Kahlmeter G., Bieber L., Runehagen A., Breider JM. Intrafamilial spread of highly virulent Staphylococcus aureus strains carrying the gene for Panton-Valentine leukocidin. Scand J Infect Dis. 34:763–764. 2002.19). Otsuka T., Saito K., Dohmae S., Takano T., Higuchi W., Takizawa Y., Okubo T., Iwakura N., Yamamoto T. Key adhesin gene in community-acquired methicillin-resistant Staphylococcus aureus. Biochem Biophys Res Commun. 346:1234–1244. 2006.20). Papanastasiou DA., Dimitracopoulos G., Drakou A., Haliotis F., Spiliopoulou I. Significant bacteriuria in infants and young children and relation to bacterial species and pyuria. Clin Microbiol Infect. 4:284–287. 1998.
Article21). Prévost G., Mourey L., Colin DA., Menestrina G. Staphylococcal pore-forming toxins. Curr Top Microbiol Immunol. 257:53–83. 2001.
Article22). Saiman L., O'Keefe M., Graham PL 3rd., Wu F., Saïd-Salim B., Kreiswirth B., LaSala A., Schlievert PM., Della-Latta P. Hospital transmission of community-acquired methicillin-resistant Staphylococcus aureus among postpartum women. Clin Infect Dis. 37:1313–1319. 2003.23). Shigemura K., Tanaka K., Okada H., Nakano Y., Kinoshita S., Gotoh A., Arakawa S., Fujisawa M. Pathogen occurrence and antimicrobial susceptibility of urinary tract infection cases during a 20-year period (1983~2002) at a single institution in Japan. Jpn J Infect Dis. 58:303–308. 2005.24). Supersac G., Prévost G., Piémont Y. Sequencing of leucocidin R from Staphylococcus aureus P83 suggests that staphylococcal leucocidins and gamma-hemolysin are members of a single, two-component family of toxins. Infect Immun. 61:580–587. 1993.25). Tristan A., Ying L., Bes M., Etienne J., Vandenesch F., Lina G. Use of multiplex PCR to identify Staphylococcus aureus adhesins involved in human hematogenous infections. J Clin Microbiol. 41:4465–4467. 2003.26). Wang CC., Lo WT., Chu ML., Siu LK. Epidemiological typing of community-acquired methicillin-resistant Staphylococcus aureus isolates from children in Taiwan. Clin Infect Dis. 39:481–487. 2004.27). Wannet WJ., Spalburg E., Heck ME., Pluister GN., Tiemersma E., Willems RJ., Huijsdens XW., de Neeling AJ., Etienne J. Emergence of virulent methicillin-resistant Staphylococcus aureus strains carrying Panton-Valentine leucocidin genes in The Netherlands. J Clin Microbiol. 43:3341–3345. 2005.28). Witte W., Strommenger B., Cuny C., Heuck D., Nuebel U. Methicillin-resistant Staphylococcus aureus containing the Panton-Valentine leucocidin gene in Germany in 2005 and 2006. J Antimicrob Chemother. 60:1258–1263. 2007.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nasal Colonization of Serine Protease esp Positive Staphylococcus epidermidis Affecting Staphylococcus aureus Colonization
- Detection of Multidrug Resistant Patterns and Associated - genes of Methicillin Resistant Staphylococcus aureus ( MRSA ) Isolated from Clinical Specimens
- Restriction Endonuclease Analysis of Plasmids and Antimicrobial Resistance Pattern of Staphylococcus Aureus and S. Epidermidis Isolated from Clinical Specimens
- Bilateral Staphylococcus Epidermidis Endophthalmitis After Cataract Extraction
- In vitro activities of eight antibiotics against methicillin-resistant S. aureus and S. epidermidis strains isolated in Korea