Immune Netw.
2009 Aug;9(4):127-132. 10.4110/in.2009.9.4.127.
Expression and Function of TLR2 on CD4 Versus CD8 T Cells
- Affiliations
-
- 1Department of Microbiology and Immunology, College of Medicine, Inje University, Busan 614-735, Korea. sseo@inje.ac.kr
- 2Department of Hemato/Oncology, College of Medicine, Inje University, Busan 614-735, Korea.
- KMID: 1474584
- DOI: http://doi.org/10.4110/in.2009.9.4.127
Abstract
- BACKGROUND
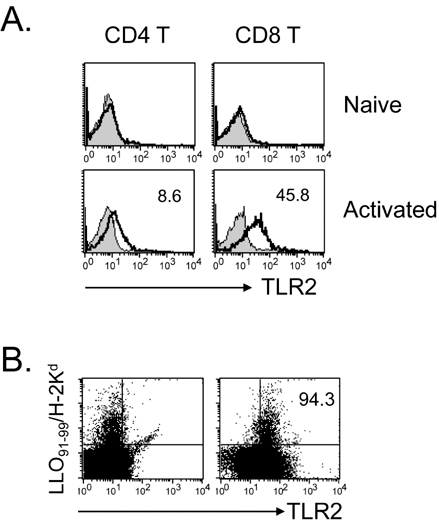
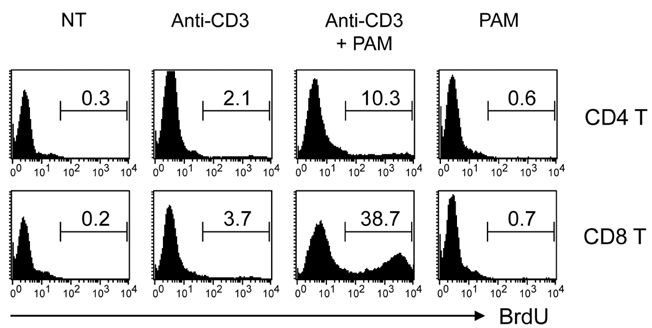
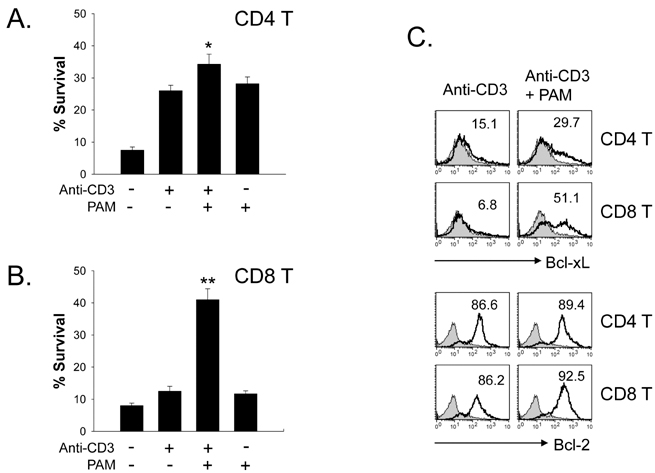
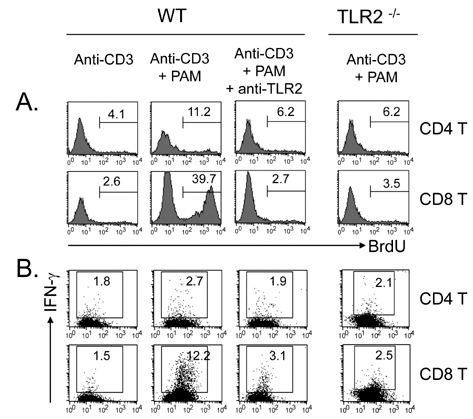
Toll-like receptors (TLRs) play a fundamental role in innate immunity through their capacity to recognize pathogen-associated molecular patterns. Also, TLRs that are expressed in T cells are reported to function as co-stimulatory receptors. However, the functional capacity of TLRs on CD4 T and CD8 T cells has not been directly compared. Here we compared CD4 and CD8 T cell responses to TLR2 ligand plus TCR-mediated stimulation. METHODS: TLR2 expression was analyzed on T cell subsets under naive and alloantigen-primed conditions. We analyzed the effects of TLR2 co-stimulation on proliferation and survival of T cell subsets in vitro when stimulated with soluble anti-CD3 in the presence or absence of synthetic ligand Pam3CSK4. RESULTS: TLR2 expression on CD8 T cells was induced following activation; this expression was much higher than on CD4 T cells. Thus, the molecule was constitutively expressed on Listeria-specific memory CD8 T cells. Based on these expression levels, proliferation and survival were markedly elevated in CD8 T cells in response to the TLR2 co-stimulation by Pam3CSK4 compared with those in CD4 T cells. CONCLUSION: Our data show that TLR2 co-stimulation is more responsible for proliferation and survival of CD8 T cells than for that of CD4 T cells.
Keyword
MeSH Terms
Figure
Reference
-
1. Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003. 21:335–376.
Article2. Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001. 1:135–145.
Article3. Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci USA. 2004. 101:3029–3034.
Article4. Gelman AE, Zhang J, Choi Y, Turka LA. Toll-like receptor ligands directly promote activated CD4+ T cell survival. J Immunol. 2004. 172:6065–6073.
Article5. Gelman AE, LaRosa DF, Zhang J, Walsh PT, Choi Y, Sunyer JO, Turka LA. The adaptor molecule MyD88 activates PI-3 kinase signaling in CD4+ T cells and enables CpG oligodeoxynucleotide-mediated costimulation. Immunity. 2006. 25:783–793.
Article6. Imanishi T, Hara H, Suzuki S, Suzuki N, Akira S, Saito T. Cutting edge: TLR2 directly triggers Th1 effector functions. J Immunol. 2007. 178:6715–6719.
Article7. Cottalorda A, Verschelde C, Marçais A, Tomkowiak M, Musette P, Uematsu S, Akira S, Marvel J, Bonnefoy-Berard N. TLR2 engagement on CD8 T cells lowers the threshold for optimal antigen-induced T cel activation. Eur J Immunol. 2006. 36:1684–1693.
Article8. Quigley M, Martinez J, Huang X, Yang Y. A critical role for direct TLR2-MyD88 signaling in CD8 T-cell clonal expansion and memory formation following vaccinia viral infection. Blood. 2009. 113:2256–2264.
Article9. Rahman AH, Cui W, Larosa DF, Taylor DK, Zhang J, Goldstein DR, Wherry EJ, Kaech SM, Turka LA. MyD88 plays a critical T cell-intrinsic role in supporting CD8 T cell expansion during acute lymphocytic choriomeningitis virus infection. J Immunol. 2008. 181:3804–3810.
Article10. Asprodites N, Zheng L, Geng D, Velasco-Gonzalez C, Sanchez-Perez L, Davila E. Engagement of Toll-like receptor-2 on cytotoxic T-lymphocytes occurs in vivo and augments antitumor activity. FASEB J. 2008. 22:3628–3637.
Article11. Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, Brown TJ, Emswiler J, Raecho H, Larsen CP, Pearson TC, Ledbetter JA, Aruffo A, Mittler RS. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997. 186:47–55.
Article12. Marsland BJ, Kopf M. Toll-like receptors: paving the path to T cell-driven autoimmunity? Curr Opin Immunol. 2007. 19:611–614.
Article13. Wong P, Pamer EG. CD8 T cell responses to infectious pathogens. Annu Rev Immunol. 2003. 21:29–70.
Article14. Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009. 27:83–117.
Article15. Wong P, Lara-Tejero M, Ploss A, Leiner I, Pamer EG. Rapid development of T cell memory. J Immunol. 2004. 172:7239–7245.
Article16. Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003. 21:685–711.
Article17. Kerfoot SM, Long EM, Hickey MJ, Andonegui G, Lapointe BM, Zanardo RC, Bonder C, James WG, Robbins SM, Kubes P. TLR4 contributes to disease-inducing mechanisms resulting in central nervous system autoimmune disease. J Immunol. 2004. 173:7070–7077.
Article18. Lee EK, Kang SM, Paik DJ, Kim JM, Youn J. Essential roles of toll-like receptor 4 signaling in arthritis induced by type II collagen antibody and LPS. Int Immunol. 2005. 17:325–333.
Article19. Meng G, Rutz M, Schiemann M, Metzger J, Grabiec A, Schwandner R, Luppa PB, Ebel F, Busch DH, Bauer S, Wagner H, Kirschning CJ. Antagonistic antibody prevents toll-like receptor 2-driven lethal shock-like syndromes. J Clin Invest. 2004. 113:1473–1481.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role for CD40 and CD40L Expression in Generating CD8 T Cell Response to Minor Histcompatibility Antigen, H60
- The effect of interleukin 2 on the induction Of Nk 1.1 expression in CD8+ and CD4-CD8-T Cell
- The Role of CD4 T Cell Help in CD8 T Cell Differentiation and Function During Chronic Infection and Cancer
- Relationship between T cell subset and clinical characteristics in bronchlal asthma
- The Expression of Programmed Death-1 in Circulating CD4+ and CD8+ T Cells during Hepatitis B Virus Infection Progression and Its Correlation with Clinical Baseline Characteristics