J Korean Med Assoc.
2009 Feb;52(2):135-142. 10.5124/jkma.2009.52.2.135.
Molecular Imaging of Angiogenesis
- Affiliations
-
- 1Department of Nuclear Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Korea. khnm.lee@samsung.com
- KMID: 1469548
- DOI: http://doi.org/10.5124/jkma.2009.52.2.135
Abstract
- Angiogenesis, the process whereby new capillaries are formed by outgrowth from existing microvessels, is required for tumor growth and metastasis, as well as for healing of ischemic injuries. Because angiogenesis is a promising target for molecular therapies, there is a real need to develop molecular imaging methods to monitor angiogenesis activity. Direct imaging of angiogenesis can help define the pathophysiology of angiogenic processes in vivo, and foster personized medicine by identifying patients likely to respond to angiogenesis-targeted drugs and accurately monitor the therapeutic efficacy. Promising imaging targets include integrins, vascular endothelial growth factor (VEGF) receptors, and matrix metalloproteinases. While MRI and optical imaging modalities are also workable, radiolabeled RGD (arginine-glycine-aspartate) probes that target alpha(v)beta(3) integrins overexpressed on activated endothelia are the most extensively investigated and successful angiogenesis imaging technique to date. This technique has repeatedly been validated in preclinical models of cancers and ischemic diseases, and clinical studies are presently ongoing to elucidate the value of RGD positron image tomography (PET) imaging in human patients. Herein, we review the current status of angiogenesis imaging research with special emphasis on integrin-targeted techniques.
Keyword
MeSH Terms
Figure
Reference
-
1. Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005. 438:967–974.
Article2. Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002. 2:727–739.
Article3. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000. 407:249–257.
Article4. Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003. 9:653–660.
Article5. Tongers J, Roncalli JG, Losordo DW. Therapeutic angiogenesis for critical limb ischemia: microvascular therapies coming of age. Circulation. 2008. 118:9–16.
Article6. Ahn A, Frishman WH, Gutwein A, Passeri J, Nelson M. Therapeutic angiogenesis: a new treatment approach for ischemic heart disease-part I. Cardiol Rev. 2008. 16:163–171.7. Provenzale JM. Imaging of angiogenesis: clinical techniques and novel imaging methods. Am J Roentgenol. 2007. 188:11–23.
Article8. McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat Med. 2003. 9:713–725.
Article9. Riggs TL. Research and development costs for drugs. Lancet. 2004. 363:184.
Article10. Cai W, Rao J, Gambhir SS, Chen X. How molecular imaging is speeding up antiangiogenic drug development. Mol Cancer Ther. 2006. 5:2624–2633.
Article11. Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003. 3:401–410.
Article12. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003. 9:669–676.
Article13. Hwang R, Varner J. The role of integrins in tumor angiogenesis. Hematol Oncol Clin North Am. 2004. 18:991–1006.
Article14. Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KCP. Detection of tumor angiogenesis in vivo by αvβ3 targeted magnetic resonance imaging. Nat Med. 1998. 4:623–626.
Article15. Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science. 1994. 264:569–571.
Article16. Haubner R, Wester HJ, Reuning U, Senekowitsch-Schmidtke R, Diefenbach B, Kessler H, Stöcklin G, Schwaiger M. Radiolabeled αvβ3 integrin antagonists: a new class of tracers for tumor targeting. J Nucl Med. 1999. 40:1061–1071.17. Haubner R, Wester HJ, Burkhart F, Senekowitsch-Schmidtke R, Weber W, Goodman SL, Kessler H, Schwaiger M. Glycosylated RGD-containing peptides: tracer for tumor targeting and angiogenesis imaging with improved biokinetics. J Nucl Med. 2001. 42:326–336.18. Haubner R, Wester HJ, Weber WA, Mang C, Ziegler SI, Goodman SL, Senekowitsch-Schmidtke R, Kessler H, Schwaiger M. Noninvasive imaging of αvβ3 integrin expression using 18F-labeled RGD-containing glyco-peptide and positron emission tomography. Cancer Res. 2001. 61:1781–1785.19. Pichler BJ, Kneilling M, Haubner R, Braumüller H, Schwaiger M, Röcken M, Weber WA. Imaging of delayed-type hypersensitivity reaction by PET and 18F-galacto-RGD. J Nucl Med. 2005. 46:184–189.20. Haubner R, Weber WA, Beer AJ, Vabuliene E, Reim D, Sarbia M, Becker KF, Goebel M, Hein R, Wester HJ, Kessler H, Schwaiger M. Noninvasive visualization of the activated αvβ3 integrin in cancer patients by positron emission tomography and [18F]galactoRGD. PLoS Med. 2005. 2:e70.21. Beer AJ, Haubner R, Goebel M, Luderschmidt S, Spilker ME, Wester HJ, Weber WA, Schwaiger M. Biodistribution and pharmacokinetics of the αvβ3 selective tracer 18F-galacto-RGD in cancer patients. J Nucl Med. 2005. 46:1333–1341.22. Beer AJ, Grosu AL, Carlsen J, Kolk A, Sarbia M, Stangier I, Watzlowik P, Wester HJ, Haubner R, Schwaiger M. [18F]galacto-RGD positron emission tomography for imaging of αvβ3 expression on the neovasculature in patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007. 13:6610–6616.
Article23. Mulder WJ, van der Schaft DW, Hautvast PA, Strijkers GJ, Koning GA, Storm G, Mayo KH, Griffioen AW, Nicolay K. Early in vivo assessment of angiostatic therapy efficacy by molecular MRI. FASEB J. 2007. 21:378–383.
Article24. Zhang C, Jugold M, Woenne EC, Lammers T, Mor-genstern B, Mueller MM, Zentgraf H, Bock M, Eisenhut M, Semmler W, Kiessling F. Specific targeting of tumor angiogenesis by RGD-conjugated ultrasmall super-paramagnetic iron oxide particles using a clinical 1.5-T magnetic resonance scanner. Cancer Res. 2007. 67:1555–1562.
Article25. Lee BC, Sung HJ, Kim JS, Jung KH, Choe YS, Lee KH, Chi DY. Synthesis of Tc-99m labeled glucosamino-Asp-cyclic (Arg-Gly-Asp-d-Phe-Lys) as a potential angiogenesis imaging agent. Bioorg Med Chem. 2007. 15:7755–7764.
Article26. Jung KH, Lee KH, Paik JY, Ko BH, Bae JS, Lee BC, Sung HJ, Kim DH, Choe YS, Chi DY. Favorable biokinetic and tumor-targeting properties of 99mTc-labeled glucosamino RGD and effect of paclitaxel therapy. J Nucl Med. 2006. 47:2000–2007.27. Lee KH, Jung KH, Song SH, Kim DH, Lee BC, Sung HJ, Han YM, Choe YS, Chi DY, Kim BT. Radiolabeled RGD uptake and αv integrin expression is enhanced in ischemic murine hindlimbs. J Nucl Med. 2005. 46:472–478.28. Hua J, Dobrucki LW, Daseghi MM, Zhang J, Bourke BN, Cavaliere P, Song J, Chow C, Jahanshad N, van Royden N, Buschmann I, Madri JA, Mendisabal M, Sinusas AJ. Noninvasive imaging of angiogenesis woth a 99mTc labeled peptide targeted at αvβ3 integrin after murine hindlimb ischemia. Circulation. 2005. 111:3255–3260.
Article29. Meoli D, Sadeghi M, Krassilnikova S, Bourke BN, Giordano FJ, Dione DP, Su H, Edwards DS, Liu S, Harris TD, Madri JA, Zaret BL, Sinusas AJ. Noninvasive imaging of myocardial angiogenesis following experimental myocardial infarction. J Clin Invest. 2004. 113:1684–1691.
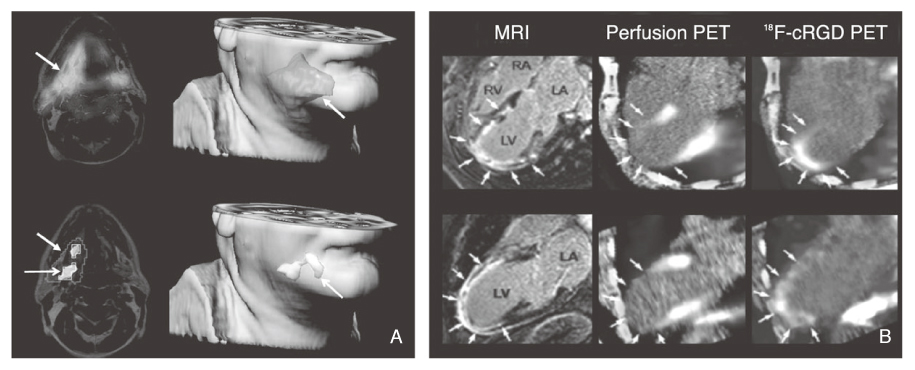
Article30. Makowski MR, Ebersberger U, Nekolla S, Schwaiger M. In vivo molecular imaging of angiogenesis, targeting alphavbeta3 integrin expression, in a patient after acute myocardial infarction. Eur Heart J. 2008. 29:2201.
Article31. Li S, Peck-Radosavljevic M, Kienast O, Preitfellner J, Hamilton G, Kurtaran A, Pirich C, Angelberger P, Dudczak R. Imaging gastrointestinal tumours using vascular endothelial growth factor-165 (VEGF165) receptor scintigraphy. Ann Oncol. 2003. 14:1274–1277.
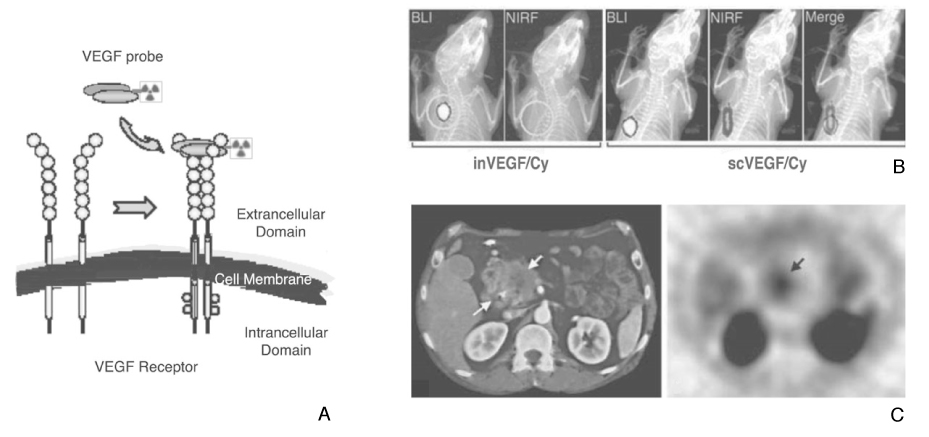
Article32. Backer MV, Levashova Z, Patel V, Jehning BT, Claffey K, Blankenberg FG, Backer JM. Molecular imaging of VEGF receptors in angiogenic vasculature with single-chain VEGF-based probes. Nat Med. 2007. 13:504–509.
Article33. Lu E, Wagner WR, Schellenberger U, Abraham JA, Klibanov AL, Woulfe SR, Csikari MM, Fischer D, Schreiner GF, Brandenburger GH, Villanueva FS. Targeted in vivo labeling of receptors for vascular endothelial growth factor: approach to identification of ischemic tissue. Circulation. 2003. 108:97–103.
Article34. Li S, Peck-Radosavljevic M, Kienast O, Preitfellner J, Havlik E, Schima W, Traub-Weidinger T, Graf S, Beheshti M, Schmid M, Angelberger P, Dudczak R. Iodine-123-vascular endothelial growth factor-165 (123I-VEGF165). Biodistribution, safety and radiation dosimetry in patients with pancreatic carcinoma. Q J Nucl Med Mol Imaging. 2004. 48:198–206.