J Korean Endocr Soc.
2009 Dec;24(4):223-226. 10.3803/jkes.2009.24.4.223.
Why to Keep Osteocytes Alive and How?
- Affiliations
-
- 1Professor of Anatomy and Cell Biology, Adjunct Professor of Medicine, Division of Endocrinology, Indiana University School of Medicine, Indianapolis, Indiana, USA.
- KMID: 1468538
- DOI: http://doi.org/10.3803/jkes.2009.24.4.223
Abstract
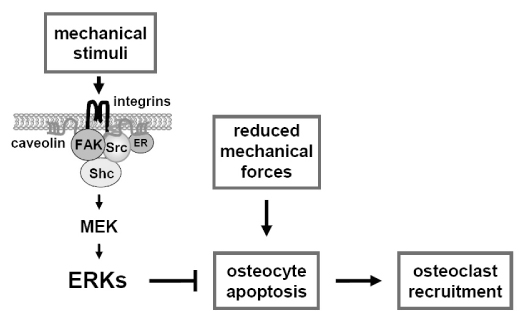
- It has been long proposed that the osteocyte network continually compares present mechanical strains to usual levels of strain, and triggers signals to osteoclasts or osteoblasts resulting in bone loss or gain, as needed. Whereas physiological levels of mechanical stimulation maintain bone mass, too low or too high levels of strain induce bone resorption. One mechanism by which osteocytes may trigger bone resorption is by undergoing apoptosis. Either low or high levels of mechanical loading lead to increased prevalence of osteocyte apoptosis, which temporally precedes and is spatially associated with osteoclast recruitment and the subsequent increase in bone resorption[1,2]. A cause and effect relationship between osteocyte death and bone resorption has been demonstrated using a transgenic mouse model of inducible osteocyte ablation in which osteocyte apoptosis was sufficient to trigger osteoclast recruitment[3]. In addition, the normal osteoclastogenic response to unloading was missing in bones from osteocyte-depleted mice, confirming that osteocytes are indispensable for the skeletal adaptation to weightlessness. Because osteocyte apoptosis is inhibited not only by mechanical stimulation but also by estrogens and bisphosphonates, these findings raise the intriguing possibility that preservation of osteocyte viability contributes to the anti-remodeling properties of these agents.
MeSH Terms
Figure
Reference
-
1. Aguirre JI, Plotkin LI, Stewart SA, Weinstein RS, Parfitt AM, Manolagas SC, Bellido T. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J Bone Miner Res. 2006. 21:605–615.2. Verborgt O, Gibson GJ, Schaffler MB. Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J Bone Miner Res. 2000. 15:60–67.3. Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007. 5:464–475.4. Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003. 22:6267–6276.5. Van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE, ten Dijke P, Lowik CW. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004. 199:805–814.6. Balemans W, Van Hul W. Human genetics of SOST. J Musculoskelet Neuronal Interact. 2006. 6:355–356.7. Ke HZ, Ominsky M, Li X, Simonet S, Lacey DL, Paszty C. Bone anabolism achieved by reducing sclerostin bioavailability with an anti-sclerostin antibody. J Musculoskelet Neuronal Interact. 2006. 6:359–360.8. Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008. 283:5866–5875.9. Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O'Brien CA, Manolagas SC, Jilka RL. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005. 146:4577–4583.10. O'Brien CA, Plotkin LI, Galli C, Goellner JJ, Gortazar AR, Allen MR, Robling AG, Bouxsein M, Schipani E, Turner CH, Jilka RL, Weinstein RS, Manolagas SC, Bellido T. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS One. 2008. 3:e2942.11. Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998. 102:274–282.12. Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001. 104:719–730.13. Tomkinson A, Reeve J, Shaw RW, Noble BS. The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J Clin Endocrinol Metab. 1997. 82:3128–3135.14. Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999. 104:1363–1374.15. Plotkin LI, Mathov I, Aguirre JI, Parfitt AM, Manolagas SC, Bellido T. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases, and ERKs. Am J Physiol Cell Physiol. 2005. 289:C633–C643.16. Bakker A, Klein-Nulend J, Burger E. Shear stress inhibits while disuse promotes osteocyte apoptosis. Biochem Biophys Res Commun. 2004. 320:1163–1168.17. Marcus R. Bilezikian JP, Raisz LG, Rodan GA, editors. Mechanisms of exercise effects on bone. Principles of bone biology. 2002. San Diego: Academic Press;1477–1488.18. Bikle DD, Halloran BP, Morey-Holton E. Spaceflight and the skeleton: lessons for the earthbound. Gravit Space Biol Bull. 1997. 10:119–135.19. Kalajzic I, Braut A, Guo D, Jiang X, Kronenberg MS, Mina M, Harris MA, Harris SE, Rowe DW. Dentin matrix protein 1 expression during osteoblastic differentiation, generation of an osteocyte GFP-transgene. Bone. 2004. 35:74–82.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Predicting the Role of Osteal Macrophages and Osteocytes in Bone Tissue Network Using a Mathematical Modeling
- Recent Progress in Osteocyte Research
- Immunocytochemical Study on Vimentin Expression in Osteoblasts and Osteocytes of Rat
- Mouse Models for the Evaluation of Osteocyte Functions
- Hypoxia Inducible Factor-1alpha Directly Induces the Expression of Receptor Activator of Nuclear Factor-kappaB Ligand in MLO-Y4 Osteocytes