J Korean Surg Soc.
2009 Jul;77(1):29-36. 10.4174/jkss.2009.77.1.29.
Clinical Significance of p53, Ki-67 and Galectin-3 Expressions in Papillary Thyroid Carcinoma
- Affiliations
-
- 1Division of Breast and Endocrine Surgery, Hallym University Sacred Heart Hospital, College of Medicine, Hallym University, Anyang, Korea. lskim0503@hallym.ac.kr
- KMID: 1464939
- DOI: http://doi.org/10.4174/jkss.2009.77.1.29
Abstract
-
PURPOSE: There are few molecular markers useful in practice for predicting prognosis of papillary thyroid carcinoma (PTC) despite numerous basic researches. The objective of this study was to evaluate the prognostic values of several candidate markers of PTC (p53, Ki-67 and galectin-3) using immunohistochemistry (IHC), one of the most practical methods.
METHODS
IHC for p53, Ki-67 and galectin-3 were performed on formalin-fixed paraffin-embedded tissues of 160 PTC specimens using monoclonal antibodies. The associations of the expressions of these markers with multiple clinicopathologic prognostic factors were assessed.
RESULTS
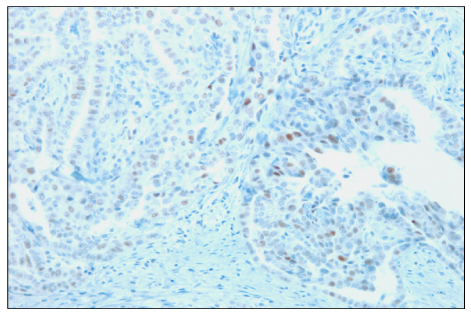
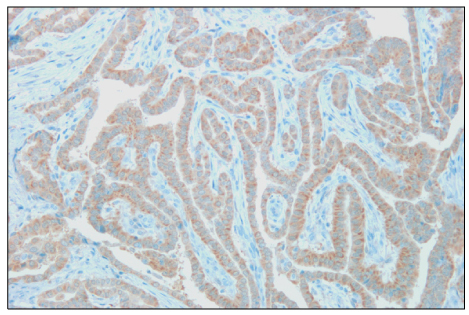
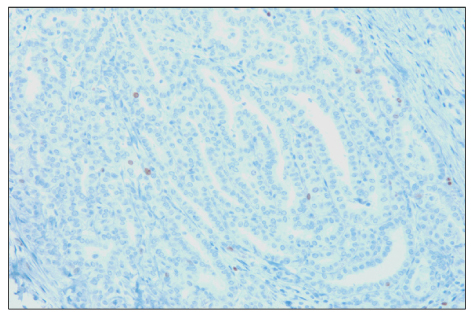
The overexpresion rates of p53, Ki-67 and galectin-3 were 48.8%, 64.3% and 97.8%, respectively. Overexpression of p53 protein was positively associated with extrathyroidal extension (P<0.001). In addition, p53 immunoreactivity was more prevalent among Ki-67 overexpressed specimens (P<0.001). Ki-67 immunoreactivity was positively correlated with tumor size (P<0.05), which became more distinct when accompanied with p53 overexpression (P<0.01). In contrast, no relationship between galectin-3 immunoreactivity and clinical prognostic factors was found.
CONCLUSION
Our results suggest that overexpression of p53 protein and Ki-67 in papillary thyroid carcinoma is associated with tumor progression and that IHC for these proteins could be useful for predicting prognosis of patients with PTC.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Prognostic Value of p53 and Cyclin D1 in Papillary Thyroid Carcinoma
Jae Yeon Seok, Dong Hae Chung, Yoo Seung Chung, Jung Won Ryu, Young Don Lee
Korean J Endocr Surg. 2015;15(2):25-33. doi: 10.16956/kjes.2015.15.2.25.
Reference
-
1. Ministry for Health, Welfare and Family Affairs. Annual Report of cancer incidence (2005) and survival (1993-2005) in Korea. 2008.2. Ain KB. Papillary thyroid carcinoma. Etiology, assessment, and therapy. Endocrinol Metab Clin North Am. 1995. 24:711–760.3. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994. 97:418–428.4. Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991. 351:453–456.5. Farid NR. P53 mutations in thyroid carcinoma: tidings from an old foe. J Endocrinol Invest. 2001. 24:536–545.6. Ito T, Seyama T, Mizuno T, Tsuyama N, Hayashi T, Hayashi Y, et al. Unique association of p53 mutations with undifferentiated but not with differentiated carcinomas of the thyroid gland. Cancer Res. 1992. 52:1369–1371.7. Park SS, Huh JR, Lee SS, Kang YK, Heo DS, Kim CW. Correlation between clinical outcome and proliferation index in diffuse large B-cell lymphoma. Korean J Pathol. 1999. 33:475–482.8. Fukumori T, Kanayama HO, Raz A. The role of galectin-3 in cancer drug resistance. Drug Resist Updat. 2007. 10:101–108.9. Yeo CJ. Tumor suppressor genes: a short review. Surgery. 1999. 125:363–366.10. Dobosz T, Lukienczuk T, Sasiadek M, Kuczynska A, Jankowska E, Blin N. Microsatellite instability in thyroid papillary carcinoma and multinodular hyperplasia. Oncology. 2000. 58:305–310.11. Nigro JM, Baker SJ, Preisinger AC, Jessup JM, Hostetter R, Cleary K, et al. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989. 342:705–708.12. Nasir A, Catalano E, Calafati S, Cantor A, Kaiser HE, Coppola D. Role of p53, CD44V6 and CD57 in differentiating between benign and malignant follicular neoplasms of the thyroid. In Vivo. 2004. 18:189–195.13. Fritsche M, Haessler C, Brandner G. Induction of nuclear accumulation of the tumor-suppressor protein p53 by DNA-damaging agents. Oncogene. 1993. 8:307–318.14. Park YK, Park HR, Chi SG, Ushigome S, Unni KK. Overexpression of p53 and absent genetic mutation in clear cell chondrosarcoma. Int J Oncol. 2001. 19:353–357.15. Matias-Guiu X, Cuatrecasas M, Musulen E, Prat J. p53 expression in anaplastic carcinomas arising from thyroid papillary carcinomas. J Clin Pathol. 1994. 47:337–339.16. Chen BK, Ohtsuki Y, Furihata M, Takeuchi T, Iwata J, Liang SB, et al. Co-overexpression of p53 protein and epidermal growth factor receptor in human papillary thyroid carcinomas correlated with lymph node metastasis, tumor size and clinicopathologic stage. Int J Oncol. 1999. 15:893–898.17. Dobashi Y, Sakamoto A, Sugimura H, Mernyei M, Mori M, Oyama T, et al. Overexpression of p53 as a possible prognostic factor in human thyroid carcinoma. Am J Surg Pathol. 1993. 17:375–381.18. Godballe C, Asschenfeldt P, Jorgensen KE, Bastholt L, Clausen PP, Hansen TP, et al. Prognostic factors in papillary and follicular thyroid carcinomas: p53 expression is a significant indicator of prognosis. Laryngoscope. 1998. 108:243–249.19. Hosal SA, Apel RL, Freeman JL, Azadian A, Rosen IB, LiVolsi VA, et al. Immunohistochemical Localization of p53 in Human Thyroid Neoplasms: Correlation with Biological Behavior. Endocr Pathol. 1997. 8:21–28.20. Holm R, Nesland JM. Retinoblastoma and p53 tumour suppressor gene protein expression in carcinomas of the thyroid gland. J Pathol. 1994. 172:267–272.21. Goldenberg JD, Portugal LG, Wenig BL, Ferrer K, Wu JC, Sabnani J. Well-differentiated thyroid carcinomas: p53 mutation status and microvessel density. Head Neck. 1998. 20:152–158.22. Zedenius J, Larsson C, Wallin G, Backdahl M, Aspenblad U, Hoog A, et al. Alterations of p53 and expression of WAF1/p21 in human thyroid tumors. Thyroid. 1996. 6:1–9.23. Brown DC, Gatter KC. Monoclonal antibody Ki-67: its use in histopathology. Histopathology. 1990. 17:489–503.24. Hoos A, Lewis JJ, Antonescu CR, Dudas ME, Leon L, Woodruff JM, et al. Characterization of molecular abnormalities in human fibroblastic neoplasms: a model for genotype-phenotype association in soft tissue tumors. Cancer Res. 2001. 61:3171–3175.25. Hoos A, Stojadinovic A, Mastorides S, Urist MJ, Polsky D, Di Como CJ, et al. High Ki-67 proliferative index predicts disease specific survival in patients with high-risk soft tissue sarcomas. Cancer. 2001. 92:869–874.26. Molino A, Micciolo R, Turazza M, Bonetti F, Piubello Q, Bonetti A, et al. Ki-67 immunostaining in 322 primary breast cancers: associations with clinical and pathological variables and prognosis. Int J Cancer. 1997. 74:433–437.27. Carr K, Heffess C, Jin L, Lloyd RV. Immunohistochemical analysis of the thyroid carcinoma initializing antibodies to p53 and Ki-67. Appl Immunohistochem. 1993. 1:201–207.28. Yoshii T, Inohara H, Takenaka Y, Honjo Y, Akahani S, Nomura T, et al. Galectin-3 maintains the transformed phenotype of thyroid papillary carcinoma cells. Int J Oncol. 2001. 18:787–792.29. Kim JH, Han DH, Oh SJ, Rho YS, Ahn HY, Shin HS, et al. Clinical significance of the expression of galectin-3 in thyroid tumor. Korean J Otolaryngol - Head Neck Sur. 2006. 49:812–816.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Usefulness of Galectin-3, Cytokeratin 19, p53, and Ki-67 for the Differential Diagnosis of Thyroid Tumors
- Preferential Expression of CD44 in Thyroid Papillary Carcinoma
- The Expressions of CK7, CK20, Vimentin, p53 and Ki-67 in Papillary Thyroid Carcinoma
- Clinical Significance of the Expression of Galectin-3 in Thyroid Tumor
- Anaplastic Transformation of Papillary Thyroid Carcinoma in a Young Man: A Case Study with Immunohistochemical and BRAF Analysis