J Korean Soc Spine Surg.
2010 Jun;17(2):57-65. 10.4184/jkss.2010.17.2.57.
Adequate Serial Monolayer Passage Number of Human Intervertebral Disc Cells for Cell Therapy: Growth and Phenotype of Cells
- Affiliations
-
- 1Department of Orthopaedic Surgery, School of Medicine, Hallym University, Anyang, Korea. swkim@hallym.or.kr
- KMID: 1459269
- DOI: http://doi.org/10.4184/jkss.2010.17.2.57
Abstract
- STUDY DESIGN: This is an in-vitro experimental study.
OBJECTIVES
We wanted to analyze the changes in the growth and phenotype of human degenerative intervertebral disc cells depending on the frequency of subculture in an in vitro monolayer culture system. SUMMARY OF THE LITERATURE REVIEW: A subculture of disc cells is needed to obtain an adequate amount of disc cells for cell therapy, tissue engineering and analysis of the biological characteristics of degenerative disc cells.
MATERIALS AND METHODS
The obtained intervertebral discs were divided into the nucleus pulposus (NP) and the annulus fibrosus (AF). The AF and NP cells were cultured in a monolayer manner, respectively. At each subculture time, we analyzed the morphological changes, the adhesion rate, the proliferation rate and the viability. The expressions of types I and II collagen and proteoglycan were analyzed at the mRNA gene level.
RESULTS
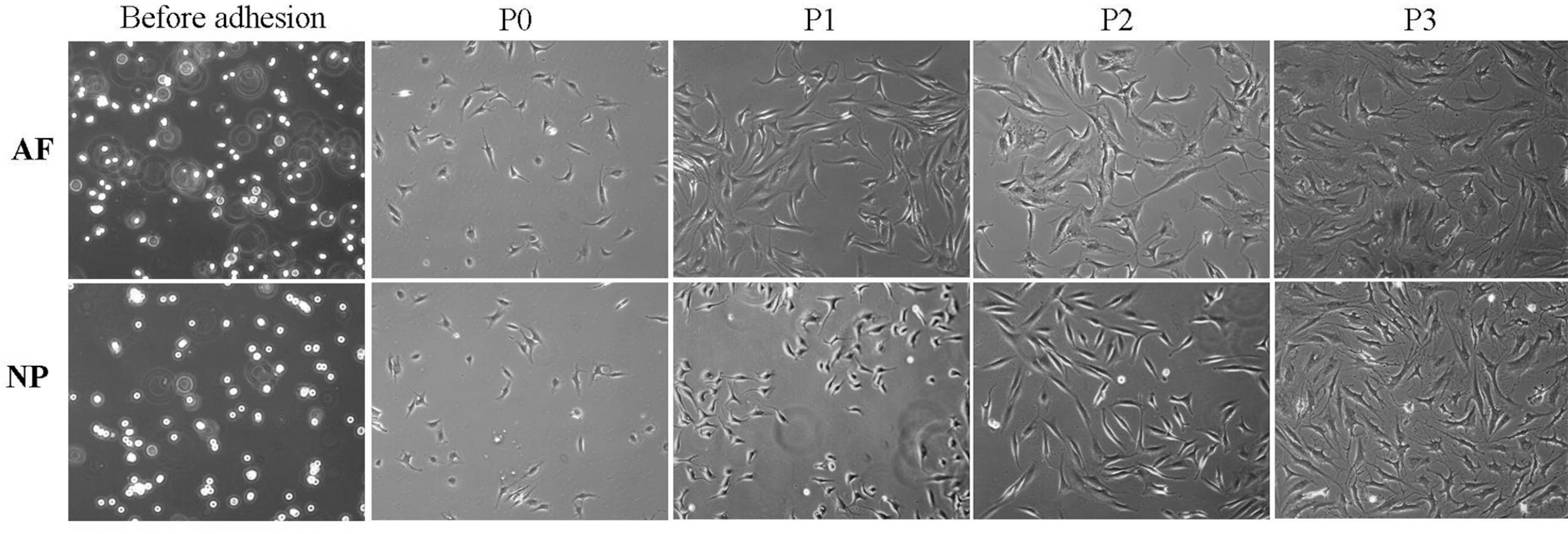
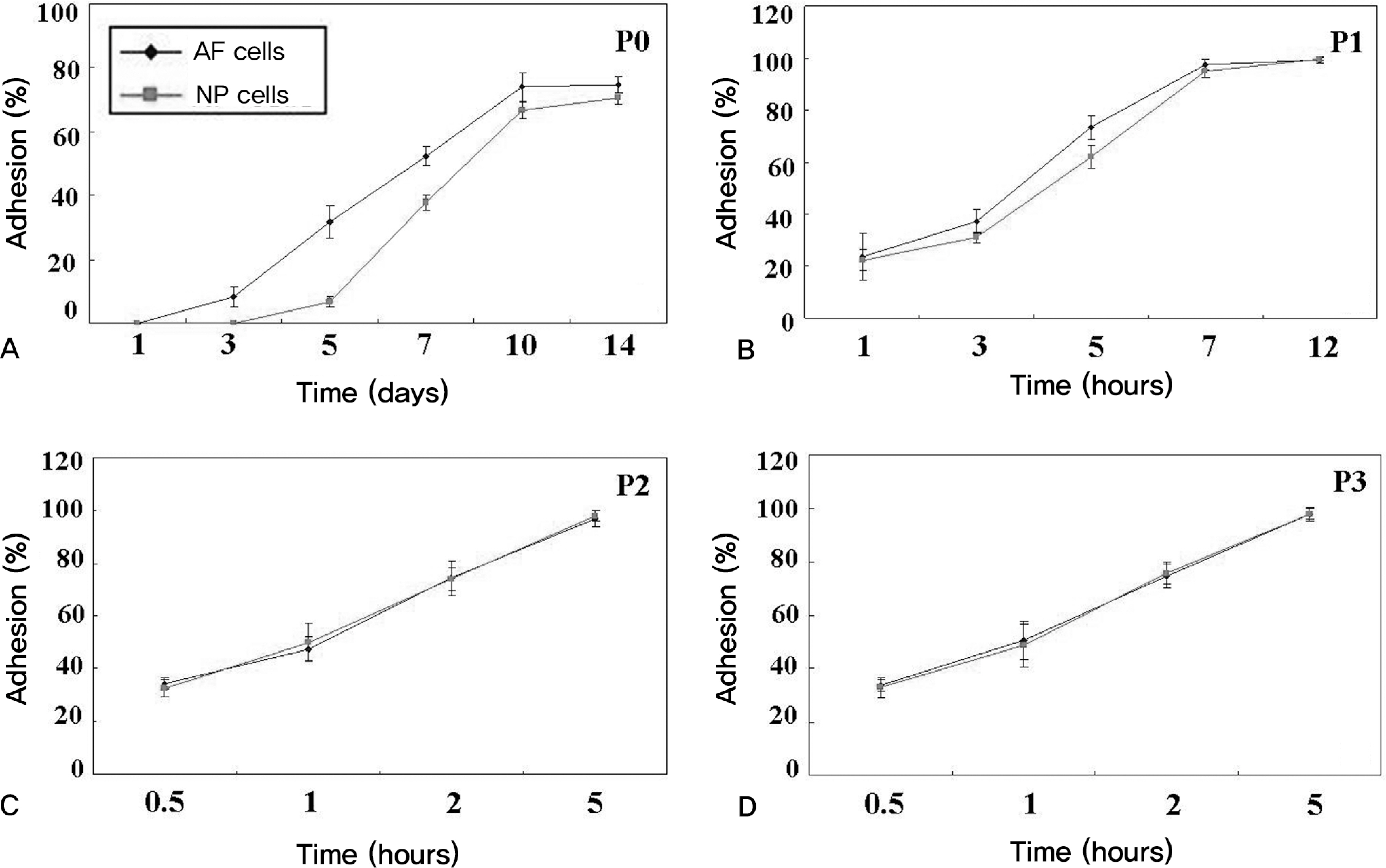
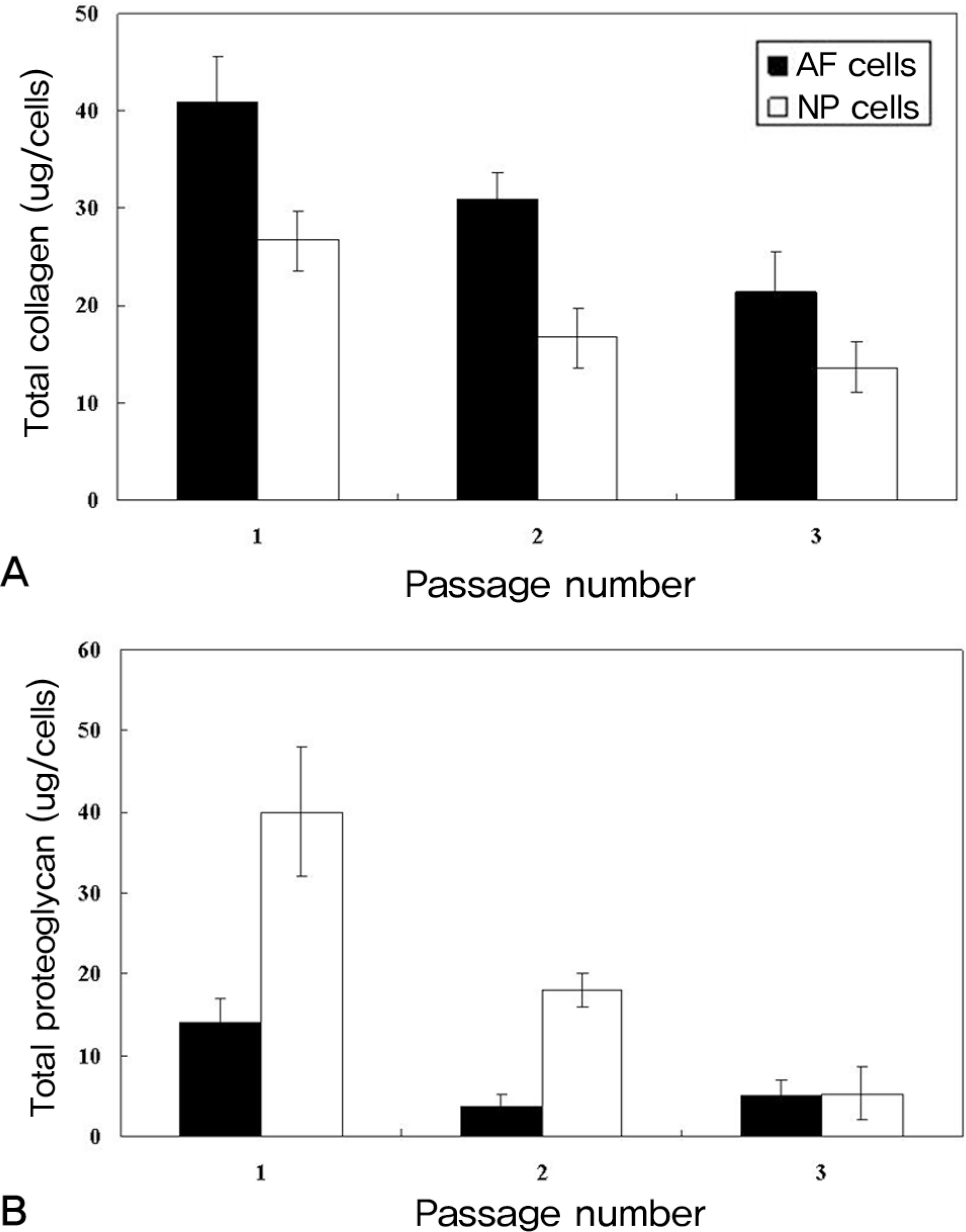
Both the AF and NP cells gradually showed a fibroblast-like spindle shape while undergoing subculture. The adhesion rate was higher at the second and third times of subculture. The cell proliferation was the highest at the second subculture time. The viability was markedly lower prior to the subculture. On RT-PCR, the type II collagen expression was gradually decreased in the NP cells. In the AF cells, Type II collagen was not expressed from the second time of subculture. The expression of proteoglycan was gradually decreased in both.
CONCLUSIONS
Following the 3rd subculture, the degenerative disc cells had completely changed their original growth and phenotypic characteristics. Therefore, we believe that it is not desirable for us to do passage cultures more than three times for cell therapy.
MeSH Terms
Figure
Reference
-
1.Urban JP., Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003. 5:120–30.2.Freemont AJ., Watkins A., Le Maitre C., Jeziorska M., Hoyland JA. Current understanding of cellular and molecular events in intervertebral disc degeneration: implications for therapy. J Pathol. 2002. 196:374–9.
Article3.Hoppenfeld S. Percutaneous removal of herniated lumbar discs. 50 cases with ten-year follow-up periods. Clin Orthop Relat Res. 1989. 238:92–7.4.Yeung AT., Tsou PM. Posterolateral endoscopic excision for lumbar disc herniation: Surgical technique, outcome, and complications in 307 consecutive cases. Spine. 2002. 27:722–31.5.White AH., von Rogov P., Zucherman J., Heiden D.Lumbar laminectomy for herniated disc: a prospective controlled comparison with internal fixation fusion. Spine. 1987. 12:305–7.6.Eie N. Comparison of the results in patients operated upon for ruptured lumbar discs with and without spinal fusion. Acta Neurochir. 1978. 41:107–13.
Article7.Eie N., Solgaard T., Kleppe H. The knee-elbow position in lumbar disc surgery: a review of complications. Spine. 1983. 8:897–900.
Article8.Freeman BJ., Davenport J. Total disc replacement in the lumbar spine: a systematic review of the literature. Eur Spine J. 2006. 15:439–47.
Article9.An HS., Thonar EJ., Masuda K. Biological repair of intervertebral disc. Spine. 2003. 28:86–92.
Article10.Ganey TM., Meisel HJ. A potential role for cell-based therapeutics in the treatment of intervertebral disc herniation. Eur Spine J. 2002. 11:206–14.
Article11.Gan JC., Ducheyne P., Vresilovic EJ., Swaim W., Shapiro IM. Intervertebral disc tissue engineering I: characterization of the nucleus pulposus. Clin Orthop Relat Res. 2003. 411:305–14.
Article12.Yoon ST. Molecular therapy of the intervertebral disc. Spine J. 2005. 5:280–6.
Article13.Baums MH., Heidrich G., Schultz W., Steckel H., Kahl E., Klinger HM. Autologous chondrocyte transplantation for treating cartilage defects of the talus. J Bone Joint Surg Am. 2006. 88:303–8.
Article14.Brittberg M., Lindahl A., Nilsson A., Ohlsson C., Isaksson O., Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994. 331:889–95.
Article15.Benya PD., Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982. 30:215–24.
Article16.Lemare F., Steimberg N., Le Griel C., Demignot S., Adolphe M. Dedifferentiated chondrocytes cultured in alginate beads: restoration of the differentiated phenotype and of the metabolic responses to interleukin-1beta. J Cell Physiol. 1998. 176:303–13.17.Bayliss MT., Johnstone B., ODBrien JP. 1988 Volvo award in basic science. Proteoglycan synthesis in the human intervertebral disc. Variation with age, region and pathology. Spine. 1988. 13:972–81.18.Chou AI., Bansal A., Miller GJ., Nicoll SB. The effect of serial monolayer passaging on the collagen expression profile of outer and inner anulus fibrosus cells. Spine. 2006. 31:1875–81.
Article19.Kluba T., Niemeyer T., Gaissmaier C., Grunder T. Human anulus fibrosus and nucleus pulposus cells of the intervertebral disc: effect of degeneration and culture system on cell phenotype. Spine. 2005. 30:2743–48.20.Wang JY., Baer AE., Kraus VB., Setton LA. Intervertebral disc cells exhibit differences in gene expression in alginate and monolayer culture. Spine. 2001. 26:1747–51.
Article21.Tullberg-Reinert H., Jundt G. In situ measurement of collagen synthesis by human bone cells with a sirius red-based colorimetric microassay: effects of transforming growth factor beta2 and ascorbic acid 2-phosphate. Histochem Cell Biol. 1999. 112:271–6.22.Farndale RW., Buttle DJ., Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986. 883:173–7.
Article23.Loeser RF., Sadiev S., Tan L., Goldring MB. Integrin expression by primary and immortalized human chondrocytes: evidence of a differential role for alpha1beta1 and alpha2beta1 integrins in mediating chondrocyte adhesion to types II and VI collagen. Osteoarthritis Cartilage. 2000. 8:96–105.24.Wang H., Kandel RA. Chondrocytes attach to hyaline or calcified cartilage and bone. Osteoarthritis Cartilage. 2004. 12:56–64.25.Hong Y., Gao C., Xie Y., Gong Y., Shen J. Collagen-coated polylactide microspheres as chondrocyte microcarriers. Biomaterials. 2005. 26:6305–13.
Article26.Yashiki S., Umegaki R., Kino-Oka M., Taya M. Evaluation of attachment and growth of anchorage-dependent cells on culture surfaces with type I collagen coating. J Biosci Bioeng. 2001. 92:385–8.
Article27.Eyre DR., Muir H. Quantitative analysis of types I and II collagens in human intervertebral discs at various ages. Biochim Biophys Acta. 1977. 492:29–42.
Article28.Johnstone B., Bayliss MT. The large proteoglycans of the human intervertebral disc. Changes in their biosynthesis and structure with age, topography, and pathology. Spine. 1995. 20:674–84.29.Lee JY., Hall R., Pelinkovic D, et al. New use of a three-dimensional pellet culture system for human intervertebral disc cells: initial characterization and potential use for tissue engineering. Spine. 2001. 26:2316–22.30.Sato M., Asazuma T., Ishihara M, et al. An atelocollagen honeycomb-shaped scaffold with a membrane seal (ACHMS-scaffold) for the culture of annulus fibrosus cells from an intervertebral disc. J Biomed Mater Res A. 2003. 64:248–56.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Lactic Acid Concentration on Cell Morphology and Phenotype in Cultured Intervertebral Disc Cell of Rabbit
- The Production of Interleukin - 1 and Stromelysin in the Cultured Intervertebral Disc Cells of Rabbit
- Biological Effect of TGF - B 1 on Human Intervertebral Disc by Cell Culture System
- The Production of Phospholipase A2 in Different Types of Cultured Human Intervertebral Disc Cells
- Osteogenic Potential of Human Mesenchymal Stem Cells During Serial Subculture