Lab Anim Res.
2012 Jun;28(2):141-145. 10.5625/lar.2012.28.2.141.
Monitoring of antibiotic resistance in bacteria isolated from laboratory animals
- Affiliations
-
- 1Department of Biomaterials Science, Collage of Natural Resources & Life Science, Pusan National University, Miryang, Korea. dyhwang@pusan.ac.kr
- 2Department of Laboratory Animal Resources, National Institute of Food and Drug Safety Evaluation, Korea FDA, Osong, Korea.
- KMID: 1436716
- DOI: http://doi.org/10.5625/lar.2012.28.2.141
Abstract
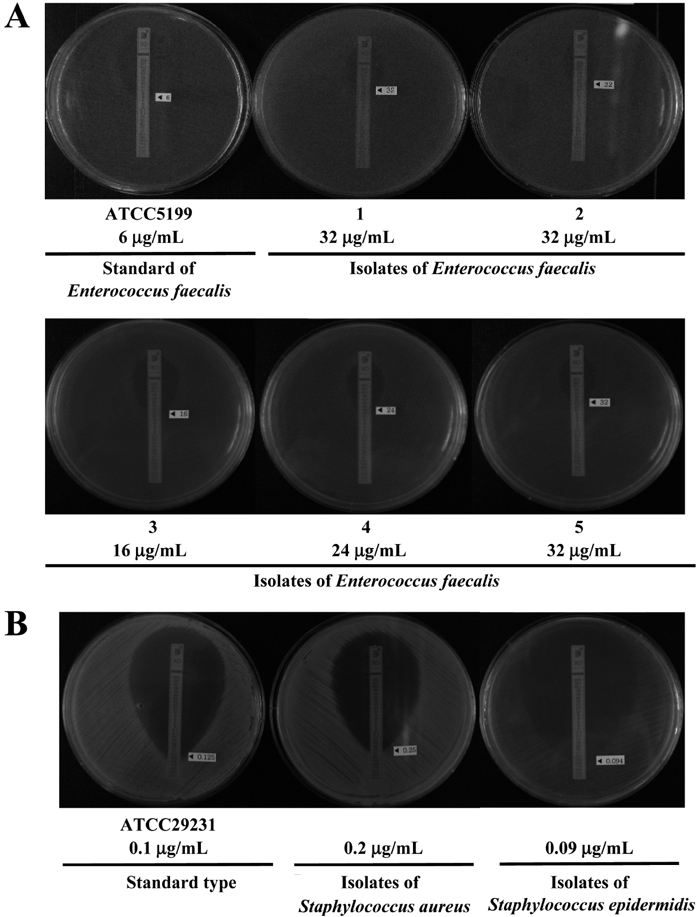
- The drug resistance of microorganisms isolated from laboratory animals never treated with antibiotics is being reported consistently, while the number of laboratory animals used in medicine, pharmacy, veterinary medicine, agriculture, nutrition, and environmental and health science has increased rapidly in Korea. Therefore, this study examined the development of antimicrobial resistance in bacteria isolated from laboratory animals bred in Korea. A total of 443 isolates (7 species) containing 5 Sphingomonas paucimobilis, 206 Escherichia coli, 60 Staphylococcus aureus, 15 Staphylococcus epidermidis, 77 Enterococcus faecalis, 27 Citrobacter freundii, 35 Acinetobacter baumannii were collected from the nose, intestine, bronchus and reproductive organs of ICR mice and SD rats. Of these species, Acinetobacter baumannii and Enterococcus faecalis showed significant antimicrobial resistance according to the minimum inhibition concentration (MIC) in E-test. In case of Acinetobacter baumannii, several isolates showed MIC values 16-128 microg/mL for cefazolin and cefoxitin, and higher resistance (128-512 microg/mL) to nitrofurantoin than that of standard type. Resistance to cefazolin, cefoxitin and nitrofurantoin was detected in 17.14, 20.00, and 8.57% of the Acinetobacter baumannii isolates, respectively. In addition, 44.1% of the Enterococcus faecalis isolates collected from the laboratory animals were resistant to oxacillin concentration of 16-32 microg/mL range, while MIC value of standard type was below oxacillin concentration of 6 microg/mL. These results suggest that in rodent species of laboratory animals, Acinetobacter baumannii are resistance to cefazolin, cefoxitin and nitrofurantoin, whereas those of Enterococcus faecalis were resistance to oxacillin.
MeSH Terms
-
Acinetobacter baumannii
Agriculture
Animals
Animals, Laboratory
Anti-Bacterial Agents
Bacteria
Bronchi
Cefazolin
Cefoxitin
Citrobacter freundii
Drug Resistance
Drug Resistance, Microbial
Enterococcus faecalis
Escherichia coli
Intestines
Korea
Mice
Mice, Inbred ICR
Nitrofurantoin
Nose
Oxacillin
Pharmacy
Rats
Rodentia
Sphingomonas
Staphylococcus aureus
Staphylococcus epidermidis
Veterinary Medicine
Anti-Bacterial Agents
Cefazolin
Cefoxitin
Nitrofurantoin
Oxacillin
Figure
Cited by 1 articles
-
Antimicrobial resistance profiles of vancomycin-resistant Enterococcus species isolated from laboratory mice
Hitoki Yamanaka, Ryuki Kadomatsu, Toshikazu Takagi, Makiko Ohsawa, Naoto Yamamoto, Noriaki Kubo, Takahira Takemoto, Kazutaka Ohsawa
J Vet Sci. 2019;20(2):. doi: 10.4142/jvs.2019.20.e13.
Reference
-
1. DuPont HL, Steele JH. Use of antimicrobial agents in animal feeds: implications for human health. Rev Infect Dis. 1987. 9(3):447–460.2. Kruse H, Sorum H. Transfer of multiple drug resistance plasmids between bacteria of diverse origins in natural microenvironments. Appl Environ Microbiol. 1994. 60(11):4015–4021.3. Maejima K, Urano T, Tamura H, Terakado N. Drug resistance of organisms isolated from feces of laboratory mice and rats. Jikken Dobutsu. 1980. 29(1):71–75.4. Hansen AK, Velschow S. Antibiotic resistance in bacterial isolates from laboratory animal colonies naive to antibiotic treatment. Lab Anim. 2000. 34(4):413–422.5. Baker CN, Banerjee SN, Tenover FC. Evaluation of Alamar colorimetric MIC method for antimicrobial susceptibility testing of gram-negative bacteria. J Clin Microbiol. 1994. 32(5):1261–1267.6. Song JY, Cheong HJ, Choi WS, Heo JY, Noh JY, Kim WJ. Clinical and microbiological characterization of carbapenem-resistant Acinetobacter baumannii bloodstream infections. J Med Microbiol. 2011. 60(5):605–611.7. Sullivan DR, Shields J, Netzer G. Fatal case of multi-drug resistant Acinetobacter baumannii necrotizing fasciitis. Am Surg. 2010. 76(6):651–653.8. Hamouda A, Findlay J, Al Hassan L, Amyes SG. Epidemiology of Acinetobacter baumannii of animal origin. Int J Antimicrob Agents. 2011. 38(4):314–318.9. Rocas IN, Siqueira JF Jr, Santos KR. Association of Enterococcus faecalis with different forms of periradicular diseases. J Endod. 2004. 30(5):315–320.10. Amyes SG. Enterococci and streptococci. Int J Antimicrob Agents. 2007. 29:Suppl 3. S43–S52.11. Courvalin P. Vancomycin resistance in gram-positive cocci. Clin Infect Dis. 2006. 42:Suppl 1. S25–S34.12. Arias CA, Contreras GA, Murray BE. Management of multidrug-resistant enterococcal infections. Clin Microbiol Infect. 2010. 16(6):555–562.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Impact of the Antibiotic Burden on the Selection of its Resistance among Gram Negative Bacteria Isolated from Children
- The Impact of Antibiotic Burden on the Selective Resistance of Gram Negative Bacteria in Children
- High-level mupirocin resistance in Gram-positive bacteria isolated from diseased companion animals
- Extended Spectrum-beta-Lactamase or Carbapenemase Producing Bacteria Isolated from Patients with Acute Cholangitis
- Comparison of antibiotic resistance profiles for Escherichia coli isolated from wild boar and domestic pig fecal samples