J Korean Med Sci.
2012 Jun;27(6):605-613. 10.3346/jkms.2012.27.6.605.
Changes of Gene Expression after Bone Marrow Cell Transfusion in Rats with Monocrotaline-Induced Pulmonary Hypertension
- Affiliations
-
- 1Department of Thoracic and Cardiovascular Surgery, Ewha Womans University, Seoul, Korea.
- 2Department of Pediatrics, Ewha Womans University, Seoul, Korea. hongym@chollian.net
- 3Department of Pathology, Ewha Womans University, Seoul, Korea.
- KMID: 1421611
- DOI: http://doi.org/10.3346/jkms.2012.27.6.605
Abstract
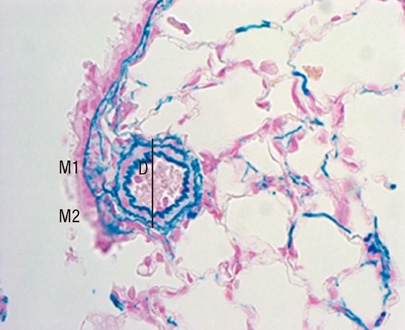
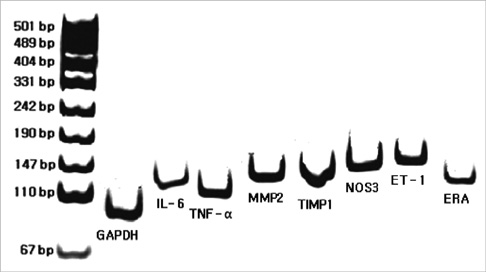
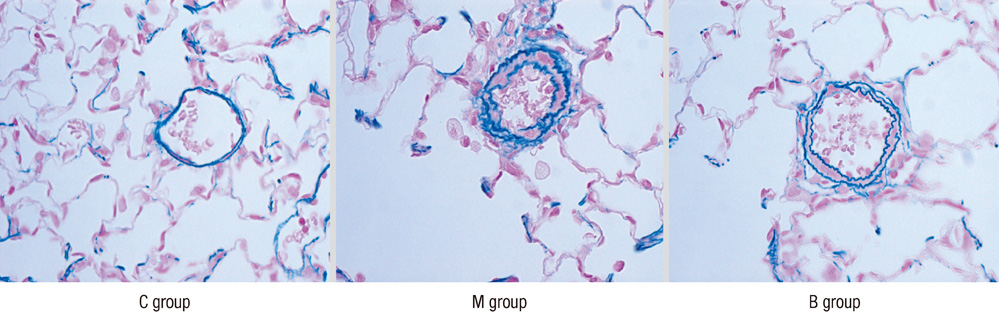
- Pulmonary artery hypertension (PAH) causes right ventricular failure and possibly even death by a progressive increase in pulmonary vascular resistance. Bone marrow-derived mesenchymal stem cell therapy has provided an alternative treatment for ailments of various organs by promoting cell regeneration at the site of pathology. The purpose of this study was to investigate changes of pulmonary haemodynamics, pathology and expressions of various genes, including ET (endothelin)-1, ET receptor A (ERA), endothelial nitric oxide synthase (NOS) 3, matrix metalloproteinase (MMP) 2, tissue inhibitor of matrix metalloproteinase (TIMP), interleukin (IL)-6 and tumor necrosis factor (TNF)-alpha in monocrotaline (MCT)-induced PAH rat models after bone marrow cell (BMC) transfusion. The rats were grouped as the control (C) group, monocrotaline (M) group, and BMC transfusion (B) group. M and B groups received subcutaneous (sc) injection of MCT (60 mg/kg). BMCs were transfused by intravenous injection at the tail 1 week after MCT injection in B group. Results showed that the average RV pressure significantly decreased in the B group compared with the M group. RV weight and the ratio of RH/LH+septum significantly decreased in the B group compared to the M group. Gene expressions of ET-1, ERA, NOS 3, MMP 2, TIMP, IL-6, and TNF-alpha significantly decreased in week 4 in the B group compared with the M group. In conclusion, BMC transfusion appears to improve survival rate, RVH, and mean RV pressure, and decreases gene expressions of ET-1, ERA, NOS 3, MMP 2, TIMP, IL-6, and TNF-alpha.
MeSH Terms
-
Animals
Bone Marrow Cells/*cytology
*Bone Marrow Transplantation
Cytokines/genetics/metabolism
Enzymes/genetics/metabolism
Gene Expression Regulation
Hypertension, Pulmonary/chemically induced/*metabolism/pathology
Lung/metabolism
Male
Monocrotaline/toxicity
Pulmonary Artery/physiology
Rats
Rats, Sprague-Dawley
Survival Rate
Ventricular Function/physiology
Cytokines
Enzymes
Monocrotaline
Figure
Reference
-
1. Gabbay E, Fraser J, McNeil K. Review of bosentan in the management of pulmonary arterial hypertension. Vasc Health Risk Manag. 2007. 3:887–900.2. Dandel M, Kemper D, Weng Y, Hummel M, Mulahasanovic S, Kapell S, Lehmkuhl H, Hetzer R. Primary pulmonary hypertension: survival benefits of therapy with prostacyclin analogs and transplantation. Transplant Proc. 2003. 35:2117–2120.3. McLaughlin VV, Genthner DE, Panella MM, Rich S. Reduction in pulmonary vascular resistance with long-term epoprostenol (prostacyclin) therapy in primary pulmonary hypertension. N Engl J Med. 1998. 338:273–277.4. Baber SR, Deng W, Master RG, Bunnell BA, Taylor BK, Murthy SN, Hyman AL, Kadowitz PJ. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2007. 292:H1120–H1128.5. Champion HC, Bivalacqua TJ, Greenberg SS, Giles TD, Hyman AL, Kadowitz PJ. Adenoviral gene transfer of endothelial nitric-oxide synthase (eNOS) partially restores normal pulmonary arterial pressure in eNOS-deficient mice. Proc Natl Acad Sci U S A. 2002. 99:13248–13253.6. Nagaya N, Yokoyama C, Kyotani S, Shimonishi M, Morishita R, Uematsu M, Nishikimi T, Nakanishi N, Ogihara T, Yamagishi M, et al. Gene transfer of human prostacyclin synthase ameliorates monocrotaline-induced pulmonary hypertension in rats. Circulation. 2000. 102:2005–2010.7. Campbell AI, Kuliszewski MA, Stewart DJ. Cell-based gene transfer to the pulmonary vasculature. Endothelial nitric oxide synthase overexpression inhibits monocrotaline-induced pulmonary hypertension. Am J Respir Cell Mol Biol. 1999. 21:567–575.8. Campbell AI, Zhao Y, Sandhu R, Stewart DJ. Cell-based gene transfer of vascular endothelial growth factor attenuates monocrotaline induced pulmonary hypertension. Circulation. 2001. 104:2242–2248.9. Nagaya N, Kangawa K, Kanda M, Uematsu M, Horio T, Fukuyama N, Hino J, Harada-Shiba M, Okumura H, Tabata Y, et al. Hybrid cell-gene therapy for pulmonary hypertension based on phagocytosing action of endothelial progenitor cells. Circulation. 2003. 108:889–895.10. Nagaya N, Kanagawa K. Adrenomedullin in the treatment of pulmonary hypertension. Peptides. 2004. 25:2013–2018.11. Takahashi M, Nakamura T, Toba T, Kajiwara N, Kato H, Shimizu Y. Transplantation of endothelial progenitor cells into the lung to alleviate pulmonary hypertension in dogs. Tissue Eng. 2004. 10:771–779.12. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997. 276:71–74.13. Umar S, de Visser YP, Steendijk P, Schutte CI, Laghmani el H, Wagenaar GT, Bax WH, Mantikou E, Pijnappels DA, Atsma DE, et al. Allogenic stem cell therapy improves right ventricular function by improving lung pathology in rats with pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2009. 297:H1606–H1616.14. Grove JE, Lutzko C, Priller J, Henegariu O, Theise ND, Kohn DB, Krause DS. Marrow-derived cells as vehicles for delivery of gene therapy to pulmonary epithelium. Am J Respir Cell Mol Biol. 2002. 27:645–651.15. Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2001. 128:5181–5188.16. Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001. 105:369–377.17. Lim KA, Shin JY, Cho SH, Kim KW, Han JJ, Hong YM. Effect of endothelin receptor blokade on monocrotaline-induced pulmonary hypertension in rats. Korean J Pediatr. 2009. 52:689–695.18. Ito KM, Sato M, Ushijima K, Nakai M, Ito K. Alterations of endothelium and smooth muscle function in monocrotaline-induced pulmonary hypertensive arteries. Am J Physiol Heart Circ Physiol. 2000. 279:H1786–H1795.19. Lim KA, Kim KC, Cho MS, Lee BE, Kim HS, Hong YM. Gene expression of endothelin-1 and endothelin receptor A on monocrotaline-induced pulmonary hypertension in rats after bosentan treatment. Korean Circ J. 2010. 40:459–464.20. Koo HS, Kim KC, Hong YM. Gene expression of nitric oxide synthase and matrix metalloproteinase-2 in monocrotaline-induced pulmonary hypertension in rats after bosentan treatment. Korean Circ J. 2011. 41:83–90.21. Miyauchi T, Yorikane R, Sakai S, Sakurai T, Okada M, Nishikibe M, Yano M, Yamaguchi I, Sugishita Y, Goto K. Contribution of endogenous endothelin-1 to the progression of cardiopulmonary alterations in rats with monocrotaline-induced pulmonary hypertension. Circ Res. 1993. 73:887–897.22. Itoh T, Nagaya N, Fujii T, Iwase T, Nakanishi N, Hamada K, Kanagawa K, Kimura H. A combination of oral sildenafil and beraprost ameliorates pulmonary hypertension in rats. Am J Respir Crit Care Med. 2004. 169:34–38.23. Kanki-Horimoto S, Horimoto H, Mieno S, Kishida K, Watanabe F, Furuya E, Katsumata T. Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension. Circulation. 2006. 114:I181–I185.24. Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003. 100:8407–8411.25. Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001. 169:12–20.26. Nagaya N, Fujii T, Iwase T, Ohgushi H, Itoh T, Uematsu M, Yamagishi M, Mori H, Kangawa K, Kitamura S. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocar dial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol. 2004. 287:H2670–H2676.27. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002. 418:41–49.28. Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004. 94:678–685.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gene Expression of Endothelin-1 and Endothelin Receptor A on Monocrotaline-Induced Pulmonary Hypertension in Rats After Bosentan Treatment
- An inhibitory effect of tumor necrosis factor-alpha antagonist to gene expression in monocrotaline-induced pulmonary hypertensive rats model
- Effects of Bosentan Treatment on Angiotensin Converting Enzyme in Monocrotaline Induced Pulmonary Hypertension Rats
- Gene Expressions of Nitric Oxide Synthase and Matrix Metalloproteinase-2 in Monocrotaline-Induced Pulmonary Hypertension in Rats After Bosentan Treatment
- Apoptosis and Inflammation Associated Gene Expressions in Monocrotaline-Induced Pulmonary Hypertensive Rats after Bosentan Treatment