Ann Lab Med.
2012 May;32(3):225-228. 10.3343/alm.2012.32.3.225.
The First Korean Case of Candidemia due to Candida dubliniensis
- Affiliations
-
- 1Department of Laboratory Medicine, Chung-Ang University College of Medicine, Seoul, Korea. cpworld@cau.ac.kr
- KMID: 1381690
- DOI: http://doi.org/10.3343/alm.2012.32.3.225
Abstract
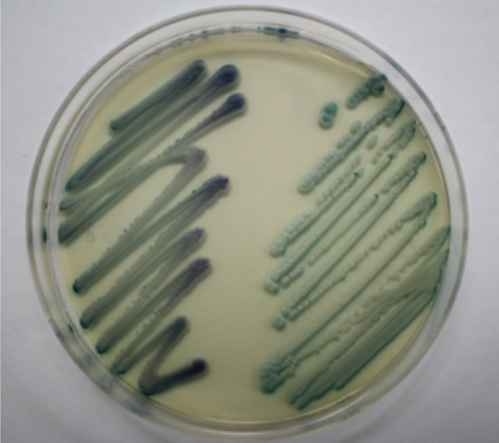
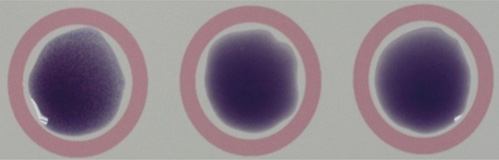
- Candidemia due to uncommon Candida spp. appears to be increasing in incidence. C. dubliniensis has been increasingly recovered from individuals not infected with HIV. Identification of C. dubliniensis can be problematic in routine clinical practice due to its phenotypic resemblance to C. albicans. We report the first case of C. dubliniensis candidemia in Korea, which occurred in a 64-yr-old woman who presented with partial seizure, drowsiness, and recurrent fever. Germ-tube positive yeast that was isolated from blood and central venous catheter tip cultures formed smooth, white colonies on sheep blood agar and Sabouraud agar plates, indicative of Candida spp. C. dubliniensis was identified using the Vitek 2 system (bioMerieux, USA), latex agglutination, chromogenic agar, and multiplex PCR. The blood isolate was susceptible to flucytosine, fluconazole, voriconazole, and amphotericin B. After removal of the central venous catheter and initiation of fluconazole treatment, the patient's condition gradually improved, and she was cleared for discharge from our hospital. Both clinicians and microbiologists should be aware of predisposing factors to C. dubliniensis candidemia in order to promote early diagnosis and appropriate treatment.
MeSH Terms
-
Amphotericin B/pharmacology
Antifungal Agents/pharmacology/therapeutic use
Candida/drug effects/*isolation & purification
Candidemia/*diagnosis/drug therapy
Catheterization, Central Venous
Female
Fluconazole/pharmacology/therapeutic use
Flucytosine/pharmacology
Humans
Microbial Sensitivity Tests
Middle Aged
Pyrimidines/pharmacology
Triazoles/pharmacology
Figure
Reference
-
1. Alangaden GJ. Nosocomial fungal infections: epidemiology, infection control, and prevention. Infect Dis Clin North Am. 2011. 25:201–225.
Article2. Arendrup MC, Bruun B, Christensen JJ, Fuursted K, Johansen HK, Kjaeldgaard P, et al. National surveillance of fungemia in Denmark (2004 to 2009). J Clin Microbiol. 2011. 49:325–334.
Article3. Bouza E, Muñoz P. Epidemiology of candidemia in intensive care units. Int J Antimicrob Agents. 2008. 32(Suppl 2):S87–S91.
Article4. Chen SC, Marriott D, Playford EG, Nguyen Q, Ellis D, Meyer W, et al. Candidaemia with uncommon Candida species: predisposing factors, outcome, antifungal susceptibility, and implications for management. Clin Microbiol Infect. 2009. 15:662–669.5. Sullivan D, Coleman D. Candida dubliniensis: characteristics and identification. J Clin Microbiol. 1998. 36:329–334.6. Mubareka S, Vinh DC, Sanche SE. Candida dubliniensis bloodstream infection: a fatal case in a lung transplant recipient. Transpl Infect Dis. 2005. 7:146–149.7. Brandt ME, Harrison LH, Pass M, Sofair AN, Huie S, Li RK, et al. Candida dubliniensis fungemia: the first four cases in North America. Emerg Infect Dis. 2000. 6:46–49.8. Gutiérrez J, Morales P, González MA, Quindós G. Candida dubliniensis, a new fungal pathogen. J Basic Microbiol. 2002. 42:207–227.9. Sahand IH, Moragues MD, Robert R, Quindos G, Ponton J. Evaluation of Bichro-Dubli Fumouze to distinguish Candida dubliniensis from Candida albicans. Diagn Microbiol Infect Dis. 2006. 55:165–167.10. Lee MK, Kim HR, Lee YJ. Identification of candida species by multiplex polymerase chain reaction. Korean J Clin Microbiol. 2006. 9:119–124.11. Dimopoulos G, Ntziora F, Rachiotis G, Armaganidis A, Falagas ME. Candida albicans versus non-albicans intensive care unit-acquired bloodstream infections: differences in risk factors and outcome. Anesth Analg. 2008. 106:523–529.12. Zaoutis TE, Prasad PA, Localio AR, Coffin SE, Bell LM, Walsh TJ, et al. Risk factors and predictors for candidemia in pediatric intensive care unit patients: implications for prevention. Clin Infect Dis. 2010. 51:e38–e45.
Article13. Asmundsdottir LR, Erlendsdottir H, Haraldsson G, Guo H, Xu J, Gottfredsson M. Molecular epidemiology of candidemia: evidence of clusters of smoldering nosocomial infections. Clin Infect Dis. 2008. 47:e17–e24.
Article14. Odds FC, Hanson MF, Davidson AD, Jacobsen MD, Wright P, Whyte JA, et al. One year prospective survey of Candida bloodstream infections in Scotland. J Med Microbiol. 2007. 56:1066–1075.15. Sullivan DJ, Moran GP, Pinjon E, Al-Mosaid A, Stokes C, Vaughan C, et al. Comparison of the epidemiology, drug resistance mechanisms, and virulence of Candida dubliniensis and Candida albicans. FEMS Yeast Res. 2004. 4:369–376.16. Odds FC, Van Nuffel L, Dams G. Prevalence of Candida dubliniensis isolates in a yeast stock collection. J Clin Microbiol. 1998. 36:2869–2873.17. Jabra-Rizk MA, Baqui AA, Kelley JI, Falkler WA Jr, Merz WG, Meiller TF. Identification of Candida dubliniensis in a prospective study of patients in the United States. J Clin Microbiol. 1999. 37:321–326.18. Pinjon E, Sullivan D, Salkin I, Shanley D, Coleman D. Simple, inexpensive, reliable method for differentiation of Candida dubliniensis from Candida albicans. J Clin Microbiol. 1998. 36:2093–2095.19. Alves SH, Milan EP, de Laet San'tAna P, Oliveira LO, Santurio JM, Colombo AL. Hypertonic Sabouraud broth as a simple and powerful test for Candida dubliniensis screening. Diagn Microbiol Infect Dis. 2002. 43:85–86.20. Staib P, Morschhäuser J. Chlamydospore formation on Staib agar as a species-specific characteristic of Candida dubliniensis. Mycoses. 1999. 42:521–524.21. Kirkpatrick WR, Revankar SG, Mcatee RK, Lopez-Ribot JL, Fothergill AW, McCarthy DI, et al. Detection of Candida dubliniensis in oropharyngeal samples from human immunodeficiency virus-infected patients in North America by primary CHROMagar candida screening and susceptibility testing of isolates. J Clin Microbiol. 1998. 36:3007–3012.22. Innings A, Ullberg M, Johansson A, Rubin CJ, Noreus N, Isaksson M, et al. Multiplex real-time PCR targeting the RNase P RNA gene for detection and identification of Candida species in blood. J Clin Microbiol. 2007. 45:874–880.23. Kim TH, Park BR, Kim HR, Lee MK. Candida dubliniensis screening using the germ tube test in clinical yeast isolates and prevalence of C. dubliniensis in Korea. J Clin Lab Anal. 2010. 24:145–148.24. Shin JH. Antifungal Resistance in Yeasts and Filamentous Fungi. Infect Chemother. 2009. 41:65–71.
Article25. Shin JH, Chae MJ, Song JW, Jung SI, Cho D, Kee SJ, et al. Changes in karyotype and azole susceptibility of sequential bloodstream isolates from patients with Candida glabrata candidemia. J Clin Microbiol. 2007. 45:2385–2391.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differentiation of Candida albicans and Candida dubliniensis using Latex Agglutination Test
- Screening of Candida dubliniensis from Respiratory Samples in Korea
- Epidemiology of Candidemia in Neonates and Children: A Single Center Experience from 2001 to 2006
- Clinical and Laboratory Features of Candidemia Caused by Different Candida Species
- Clinical Significance of Pastorex Candida Antigen Assay in Patients with Candidemia